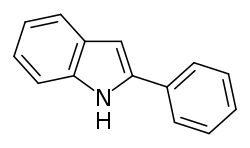
| |
| Names | |
|---|---|
| Preferred IUPAC name 2-Phenyl-1H-indole | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.012.215 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H11N |
| Molar mass | 193.249 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
2-Phenylindole is an organic compound. It is the parent structure of a group of nonsteroidal selective estrogen receptor modulators (SERMs) that includes zindoxifene, bazedoxifene, and pipendoxifene, as well as the nonsteroidal estrogen D-15414 (the major metabolite of zindoxifene).
References
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 68–69. ISBN 978-3-642-58616-3.
- International position paper on women's health and menopause : a comprehensive approach. DIANE Publishing. 2002. pp. 111–. ISBN 978-1-4289-0521-4.
- Gordon W. Gribble (9 October 2010). Heterocyclic Scaffolds II:: Reactions and Applications of Indoles. Springer Science & Business Media. pp. 14–. ISBN 978-3-642-15732-5.
This article about a heterocyclic compound is a stub. You can help Misplaced Pages by expanding it. |