| |
| Names | |
|---|---|
| Preferred IUPAC name 3-Aminobenzene-1,2-dicarboxylic acid | |
| Other names 3-Aminophthalic acid | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.024.178 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H7NO4 |
| Molar mass | 181.147 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
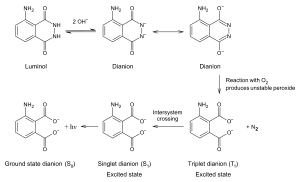
3-Aminophthalic acid is a product of the oxidation of luminol. The reaction requires the presence of a catalyst. A mixture of luminol and hydrogen peroxide is used in forensics. When the mixture is sprayed on an area that contains blood, the iron in the hemoglobin in the blood catalyzes a reaction between the mixture, resulting in 3-aminophthalate which gives out light by chemiluminescence.
References
- Harris, Tom. "How Luminol Works". HowStuffWorks. Discovery Communications. Retrieved 5 August 2013.