Pharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Purinethol, Purixan, others |
| Other names | 6-mercaptopurine (6-MP) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682653 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 5 to 37% |
| Metabolism | xanthine oxidase |
| Elimination half-life | 60 to 120 min., longer for its active metabolites |
| Excretion | kidney |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.035 |
| Chemical and physical data | |
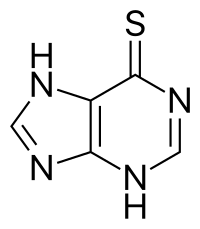
| Formula | C5H4N4S |
| Molar mass | 152.18 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Mercaptopurine (6-MP), sold under the brand name Purinethol among others, is a medication used for cancer and autoimmune diseases. Specifically it is used to treat acute lymphocytic leukemia (ALL), acute promyelocytic leukemia (APL), Crohn's disease, and ulcerative colitis. For acute lymphocytic leukemia it is generally used with methotrexate. It is taken orally.
Common side effects include bone marrow suppression, liver toxicity, vomiting, and loss of appetite. Other serious side effects include an increased risk of future cancer and pancreatitis. Those with a genetic deficiency in thiopurine S-methyltransferase are at higher risk of side effects. Use in pregnancy may harm the baby. Mercaptopurine is in the thiopurine and antimetabolite family of medications.
Mercaptopurine was approved for medical use in the United States in 1953. It is on the World Health Organization's List of Essential Medicines.
Medical uses
It is used to treat acute lymphocytic leukemia, Crohn's disease, and ulcerative colitis.
Side effects
Some of the adverse reactions of taking mercaptopurine may include diarrhea, nausea, vomiting, loss of appetite, fatigue, stomach/abdominal pain, weakness, skin rash, darkening of the skin, and hair loss. Serious adverse reactions include mouth sores, fever, sore throat, easy bruising or bleeding, pinpoint red spots on the skin, yellowing of eyes or skin, dark urine, and painful or difficult urination. Other more serious side effects include black or tarry stools (melena), bloody stools, and bloody urine. Treatment is discontinued in up to 30% of patients due these effects but therapeutic drug monitoring of the biologically active metabolites, i.e. thiopurine nucleotides can help to optimize the efficacy and safety. Clinically, most hospitals resort to on-exchange LC-MS (liquid chromatography - mass spectrometry) but the newly developed approach of porous graphitic carbon based chromatography hyphenated with mass spectrometry appears superior with respect to patient care in this respect.
Symptoms of allergic reaction to mercaptopurine include rash, itching, swelling, dizziness, trouble breathing, and inflammation of the pancreas.
In some cases, mercaptopurine may suppress the production of blood cells, both white blood cells and red blood cells. It may be toxic to bone marrow. Quarterly blood counts are necessary for people on mercaptopurine. People should stop taking the medication at least temporarily while considering alternate treatment if there is an unexplained, abnormally large drop in white blood cell count, or any other blood count.
Toxicity of mercaptopurine can be linked to genetic polymorphisms in thiopurine S-methyltransferase (TPMT), nudix hydrolase 15 (NUDT15), and inosine triphosphate pyrophosphatase (ITPA). People with specific allele variants will require dose adjustments, especially for those with homozygous variant genotypes. Large differences of TPMT and NUDT15 among ethnicities in terms of variant allele frequency should be taken into consideration in clinical practice. Caucasian people with a variant allele of the ITPA gene, experience higher rates of febrile neuropenia than people of other ethnic groups, due to differences in allelic frequencies among ethnicities.
Precautions
Mercaptopurine can lower the body's ability to fight off infection. Those taking it should get permission from a doctor to receive immunizations and vaccinations. It is also recommended that, while on the drug, one should avoid those having recently received oral polio vaccine.
This drug was formerly not recommended during pregnancy and early evidence indicated pregnant women on the drug (or the related azathioprine) showed a seven-fold incidence of fetal abnormalities as well as a 20-fold increase in miscarriage. There were also anecdotal reports linking mercaptopurine with spontaneous abortion, leading to the US FDA rating both AZA and mercaptopurine as category D drugs. However, Davis et al. 1999 found mercaptopurine, compared to methotrexate, was ineffective as a single-agent abortifacient; every woman in the mercaptopurine arm of the study had fetal cardiac activity at follow-up (two weeks later) and was given a suction abortion. A more recent, larger study, however, performed by the Cancers et Surrisque Associe aux Maladies inflamatoires intestinales En France (CESAME) indicated an overall rate of congenital malformations not significantly greater than the general population in France. The European Crohn's and Colitis Organisation (ECCO) concluded in a consensus paper in 2010 that while AZA and mercaptopurine have an FDA rating of D, new research in both animals and humans indicates that "thiopurines are safe and well tolerated during pregnancy."
Mercaptopurine causes changes to chromosomes in animals and humans, though a study in 1990 found, "while the carcinogenic potential of 6-MP cannot be precluded, it can be only very weak or marginal." Another study in 1999 noted an increased risk of developing leukemia when taking large doses of 6-MP with other cytotoxic drugs.
Drug interactions
Allopurinol inhibits xanthine oxidase, the enzyme that breaks down mercaptopurine. Those taking allopurinol (often used to prevent gout) are at risk for mercaptopurine toxicity. The dose should be reduced or allopurinol should be discontinued. Several published studies have demonstrated that the use of allopurinol in combination with low dose 6-MP helps reduce 6-MP levels, which are toxic to liver tissue, whilst increasing the therapeutic levels of 6-MP for some inflammatory conditions.
Mechanisms of action
| This section may be too technical for most readers to understand. Please help improve it to make it understandable to non-experts, without removing the technical details. (December 2014) (Learn how and when to remove this message) |
Official information from the package insert for purinethol:
- Mercaptopurine is an antimetabolite antineoplastic, as such it interferes with normal metabolic processes within cells, typically by combining with enzymes, to disrupt DNA and RNA synthesis (cell-cycle S phase-specific) leading to death of rapidly proliferating cells, especially malignant ones. Specifically, Mercaptopurine is a purine antimetabolite or purine antagonist as such inhibits DNA synthesis by inhibiting the production of the purine containing nucleotides, adenine and guanine thus halting DNA synthesis. Mercaptopurine also acts as an immunomodulator by inhibiting several pathways in nucleic acid biosynthesis preventing proliferation of cells involved in the determination and amplification of the immune response.
- Mercaptopurine (6-MP) competes with the purine derivatives hypoxanthine and guanine for the enzyme HGPRT and is itself converted to thio inosine monophosphate (TIMP).
- TIMP inhibits several chemical reactions involving inosinic acid (IMP), including the conversion of IMP to xanthylic acid (XMP) and the conversion of IMP to adenylic acid (AMP) via adenylosuccinate (SAMP).
- In addition, 6-methylthioinosinate (MTIMP) is formed by the methylation of TIMP.
- Both TIMP and MTIMP have been reported to inhibit glutamine-5-phosphoribosylpyrophosphate amidotransferase, the first enzyme unique to the de novo pathway for purine ribonucleotide synthesis. Experiments indicate that radiolabeled mercaptopurine may be recovered from the DNA in the form of deoxythioguanosine.
- Some mercaptopurine is converted to nucleotide derivatives of 6-thioguanine (6-TG) by the sequential actions of inosinate (IMP) dehydrogenase and xanthylate (XMP) aminase, converting TIMP to thioguanylic acid (TGMP).
- Animal tumors that are resistant to mercaptopurine often have lost the ability to convert mercaptopurine to TIMP. However, it is clear that resistance to mercaptopurine may be acquired by other means as well, particularly in human leukemias.
- It is not known exactly which of any one or more of the biochemical effects of mercaptopurine and its metabolites are directly or predominantly responsible for cell death.
6-MP ribonucleotide inhibits purine nucleotide synthesis and metabolism by inhibiting an enzyme called phosphoribosyl pyrophosphate amidotransferase (PRPP amidotransferase). Since this enzyme is the rate limiting factor for purine synthesis, this alters the synthesis and function of RNA and DNA. Mercaptopurine interferes with nucleotide interconversion and glycoprotein synthesis.
Pharmacogenetics
The enzyme thiopurine S-methyltransferase (TPMT) is responsible, in part, for the inactivation of 6-mercaptopurine. TPMT catalyzes the methylation of 6-mercaptopurine into the inactive metabolite 6-methylmercaptopurine – this methylation prevents mercaptopurine from further conversion into active, cytotoxic thioguanine nucleotide (TGN) metabolites. Certain genetic variations within the TPMT gene can lead to decreased or absent TPMT enzyme activity, and individuals who are homozygous or heterozygous for these types of genetic variations may have increased levels of TGN metabolites and an increased risk of severe bone marrow suppression (myelosuppression) when receiving mercaptopurine. In many ethnicities, TPMT polymorphisms that result in decreased or absent TPMT activity occur with a frequency of approximately 5%, meaning that about 0.25% of people are homozygous for these variants. However, an assay of TPMT activity in red blood cells or a TPMT genetic test can identify people with reduced TPMT activity, allowing for the adjustment of mercaptopurine dose or avoidance of the drug entirely. The FDA-approved drug label for mercaptopurine recommends testing for TPMT activity to identify people at risk for myelotoxicity. Testing for TPMT activity is an example of pharmacogenetics being translated into routine clinical care.
History
6-MP was discovered by Nobel Prize–winning scientists Gertrude B. Elion and George H. Hitchings at Burroughs Wellcome in Tuckahoe, New York, and was clinically developed in collaboration with investigators at Memorial Hospital (now Memorial Sloan Kettering Cancer Center in New York City). The collaboration was initiated by Cornelius P. Rhoads, who had run chemical weapons programs for the US Army and had been involved in the work that led to the discovery that nitrogen mustards could potentially be used as cancer drugs, and had become the director of Memorial in 1948.
References
- "Xaluprine EPAR". European Medicines Agency. 30 April 2009. Retrieved 25 June 2024.
- ^ "Mercaptopurine". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 590. ISBN 9780857111562.
- Sahasranaman S, Howard D, Roy S (August 2008). "Clinical pharmacology and pharmacogenetics of thiopurines". European Journal of Clinical Pharmacology. 64 (8): 753–67. doi:10.1007/s00228-008-0478-6. PMID 18506437. S2CID 27475772.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- "Mercaptopurine". The American Society of Health-System Pharmacists. Archived from the original on 6 September 2015. Retrieved 28 August 2015.
- Pecher D, Zelinkova Z, Lucenicova J, Peppelenbosch M, Dokupilova S, Mikusova V, et al. (November 2020). "Porous graphitic carbon based chromatography hyphenated with mass spectrometry: A new strategy for profiling thiopurine nucleotides in patients with inflammatory bowel diseases". Analytica Chimica Acta. 1137 (1137): 64–73. Bibcode:2020AcAC.1137...64P. doi:10.1016/j.aca.2020.08.064. PMID 33153610. S2CID 225287631.
- Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. (April 2015). "Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia". Journal of Clinical Oncology. 33 (11): 1235–42. doi:10.1200/jco.2014.59.4671. PMC 4375304. PMID 25624441.
{{cite journal}}: CS1 maint: overridden setting (link) - Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. (April 2016). "NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity". Nature Genetics. 48 (4): 367–73. doi:10.1038/ng.3508. PMC 5029084. PMID 26878724.
{{cite journal}}: CS1 maint: overridden setting (link) - Yin D, Xia X, Zhang J, Zhang S, Liao F, Zhang G, et al. (February 2017). "Impact of NUDT15 polymorphisms on thiopurines-induced myelotoxicity and thiopurines tolerance dose". Oncotarget. 8 (8): 13575–13585. doi:10.18632/oncotarget.14594. PMC 5355121. PMID 28088792.
{{cite journal}}: CS1 maint: overridden setting (link) - Esses SI, Morley TP (February 1983). "Spinal arachnoiditis". The Canadian Journal of Neurological Sciences. 10 (1): 2–10. doi:10.1017/s0317167100044486. PMID 6404543.
- Nørgård B, Pedersen L, Fonager K, Rasmussen SN, Sørensen HT (March 2003). "Azathioprine, mercaptopurine and birth outcome: a population-based cohort study". Alimentary Pharmacology & Therapeutics. 17 (6): 827–34. doi:10.1046/j.1365-2036.2003.01537.x. PMID 12641505. S2CID 25314258.
- Davis AR, Miller L, Tamimi H, Gown A (June 1999). "Methotrexate compared with mercaptopurine for early induced abortion". Obstetrics and Gynecology. 93 (6): 904–9. doi:10.1016/S0029-7844(98)00569-9. PMID 10362152. Archived from the original on 19 June 2006.
- Coelho J, Beaugerie L, Colombel JF, Hébuterne X, Lerebours E, Lémann M, et al. (February 2011). "Pregnancy outcome in patients with inflammatory bowel disease treated with thiopurines: cohort from the CESAME Study". Gut. 60 (2): 198–203. doi:10.1136/gut.2010.222893. PMID 21115547. S2CID 25614617.
{{cite journal}}: CS1 maint: overridden setting (link) - Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, et al. (February 2010). "The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Special situations". Journal of Crohn's & Colitis. 4 (1): 63–101. doi:10.1016/j.crohns.2009.09.009. PMID 21122490.
{{cite journal}}: CS1 maint: overridden setting (link) - Maekawa A, Nagaoka T, Onodera H, Matsushima Y, Todate A, Shibutani M, et al. (May 1990). "Two-year carcinogenicity study of 6-mercaptopurine in F344 rats". Journal of Cancer Research and Clinical Oncology. 116 (3): 245–50. doi:10.1007/BF01612898. PMID 2370249. S2CID 21675626.
{{cite journal}}: CS1 maint: overridden setting (link) - Bo J, Schrøder H, Kristinsson J, Madsen B, Szumlanski C, Weinshilboum R, et al. (September 1999). "Possible carcinogenic effect of 6-mercaptopurine on bone marrow stem cells: relation to thiopurine metabolism". Cancer. 86 (6): 1080–6. doi:10.1002/(SICI)1097-0142(19990915)86:6<1080::AID-CNCR26>3.0.CO;2-5. PMID 10491537. S2CID 45441636.
{{cite journal}}: CS1 maint: overridden setting (link) - "PURINETHOL (mercaptopurine) tablet [Gate Pharmaceuticals]" (PDF). DailyMed. Gate Pharmaceuticals. August 2012. Archived from the original on 1 January 2014. Retrieved 31 December 2013.
- "Chemotherapy".
- Nielsen OH, Vainer B, Rask-Madsen J. Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine. Aliment Pharmacol Ther. 2001 Nov;15(11):1699-708. doi: 10.1046/j.1365-2036.2001.01102.x. PMID 11683683.
- Hansen, Barbara. "Purine and Pyrimidine Metabolism." USMLE STEP 1 Biochemistry and Medical Genetics Lecture Notes. 2010 ed. N.p.: Kaplan, 2010. 288-90. Print.
- Zaza G, Cheok M, Krynetskaia N, Thorn C, Stocco G, Hebert JM, et al. (September 2010). "Thiopurine pathway". Pharmacogenetics and Genomics. 20 (9): 573–4. doi:10.1097/FPC.0b013e328334338f. PMC 3098750. PMID 19952870.
{{cite journal}}: CS1 maint: overridden setting (link) - Stocco G, Pelin M, Franca R, De Iudicibus S, Cuzzoni E, Favretto D, et al. (April 2014). "Pharmacogenetics of azathioprine in inflammatory bowel disease: a role for glutathione-S-transferase?". World Journal of Gastroenterology. 20 (13): 3534–41. doi:10.3748/wjg.v20.i13.3534. PMC 3974520. PMID 24707136.
{{cite journal}}: CS1 maint: overridden setting (link) - Fujita K, Sasaki Y (August 2007). "Pharmacogenomics in drug-metabolizing enzymes catalyzing anticancer drugs for personalized cancer chemotherapy". Current Drug Metabolism. 8 (6): 554–62. doi:10.2174/138920007781368890. PMID 17691917. Archived from the original on 12 January 2013.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. (March 2011). "Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing". Clinical Pharmacology and Therapeutics. 89 (3): 387–91. doi:10.1038/clpt.2010.320. PMC 3098761. PMID 21270794.
{{cite journal}}: CS1 maint: overridden setting (link) - Mutschler E, Schäfer-Korting M (2001). Arzneimittelwirkungen (in German) (8th ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 107, 936. ISBN 978-3-8047-1763-3.
- Payne K, Newman W, Fargher E, Tricker K, Bruce IN, Ollier WE (May 2007). "TPMT testing in rheumatology: any better than routine monitoring?". Rheumatology. 46 (5): 727–9. doi:10.1093/rheumatology/kel427. PMID 17255139.
- "Label: Mercaptopurine – mercaptopurine tablet". Archived from the original on 6 October 2015. Retrieved 11 March 2015.
- "Purixan suspension". DailyMed. 9 April 2019. Retrieved 9 April 2020.
- Wang L, Pelleymounter L, Weinshilboum R, Johnson JA, Hebert JM, Altman RB, et al. (June 2010). "Very important pharmacogene summary: thiopurine S-methyltransferase". Pharmacogenetics and Genomics. 20 (6): 401–5. doi:10.1097/FPC.0b013e3283352860. PMC 3086840. PMID 20154640.
- Bouton K (29 January 1989). "The Nobel Pair". The New York Times Magazine. Archived from the original on 7 October 2016.
- ^ Mukherjee S (2010). The Emperor of All Maladies: A Biography of Cancer. New York: Scribner. pp. 91–92. ISBN 978-1439170915.
Further reading
- Dean L (2012). "Mercaptopurine Therapy and TPMT Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520348. Bookshelf ID: NBK100660.