| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name (Prop-1-en-2-yl)benzene | |||
| Other names 2-Phenylpropene; 2-Phenylpropylene; 1-Methyl-1-phenylethylene; 1-Methyl-1-phenylethene; 1-Phenyl-1-methylethylene; 1-Phenyl-1-methylethene; (1-Methylethenyl)benzene; β-Phenylpropene; β-Phenylpropylene; α-Methylstyrol; α-Methylvinylbenzene; Isopropenylbenzene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Abbreviations | AMS | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.459 | ||
| EC Number |
| ||
| KEGG | |||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2303 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C9H10 | ||
| Molar mass | 118.179 g·mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.91 g/cm | ||
| Melting point | −24 °C (−11 °F; 249 K) | ||
| Boiling point | 165 to 169 °C (329 to 336 °F; 438 to 442 K) | ||
| Solubility in water | Insoluble | ||
| Vapor pressure | 2 mmHg (20 °C) | ||
| Magnetic susceptibility (χ) | -80.1·10 cm/mol | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |   
| ||
| Signal word | Warning | ||
| Hazard statements | H226, H319, H335, H411 | ||
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264+P265, P271, P273, P280, P303+P361+P353, P304+P340, P305+P351+P338, P319, P337+P317, P370+P378, P391, P403+P233, P403+P235, P405, P501 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 45 °C (113 °F; 318 K) | ||
| Explosive limits | 1.9–6.1% | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 4900 mg/kg (oral, rat) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | C 100 ppm (480 mg/m) | ||
| REL (Recommended) | TWA 50 ppm (240 mg/m) ST 100 ppm (485 mg/m) | ||
| IDLH (Immediate danger) | 700 ppm | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
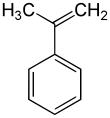
α-Methylstyrene (AMS) is an organic compound with the formula C6H5C(CH3)=CH2. It is a colorless oil.
Synthesis and reactions
AMS is formed as a by-product of the cumene process. In this procedure, cumene is converted to its radical, through a reaction with oxygen.
Normally these cumene radicals are converted to cumene hydroperoxide, however they can also undergo radical disproportionation to form AMS.
Although this is only a minor side reaction, the cumene process is run at such a large scale that the recovery of AMS is commercially viable and satisfies much of the global demand. AMS can also be produced by dehydrogenation of cumene.
The homopolymer obtained from this monomer, poly(α-methylstyrene), is unstable, being characterized by a low ceiling temperature of 65°C.
Side effects in humans
The American Conference of Governmental Industrial Hygienists (2009) defined occupational exposure limits of 10 ppm for airborne concentrations of a-methylstyrene. based on allergic reactions, and effects on the central nervous system.
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0429". National Institute for Occupational Safety and Health (NIOSH).
- "alpha-Methyl styrene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "alpha-Methylstyrene". pubchem.ncbi.nlm.nih.gov.
- James, Denis H.; Castor, William M. (2007). "Styrene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_329.pub2. ISBN 978-3527306732.
- Stevens, Malcolm P. (1999). "6". Polymer Chemistry an Introduction (3rd ed.). New York: Oxford University Press. pp. 193–194. ISBN 978-0-19-512444-6.
- Jones, G. R.; Wang, H. S.; Parkatzidis, K.; Whitfield, R.; Truong, N. P.; Anastasaki, A. (2023). "Reversed Controlled Polymerization (RCP): Depolymerization from Well-Defined Polymers to Monomers". Journal of the American Chemical Society. 145 (18): 9898–9915. doi:10.1021/jacs.3c00589. PMC 10176471. PMID 37127289.
- ^ Morgan, D. L.; Mahler, J. F.; Kirkpatrick, D. T.; Price, H. C.; O'Connor, R. W.; Wilson, R. E.; Moorman, M. P. (February 1999). "Characterization of inhaled alpha-methylstyrene vapor toxicity for B6C3F1 mice and F344 rats". Toxicological Sciences. 47 (2): 187–194. doi:10.1093/toxsci/47.2.187. ISSN 1096-6080. PMID 10220856.
- "α-METHYL STYRENE†". OSHA.