| |
| Names | |
|---|---|
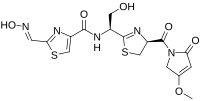
| IUPAC name N-ethyl]-2-(nitrosomethylidene)-3H-1,3-thiazole-4-carboxamide | |
| Other names Altiomycin; Matamycin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H17N5O6S2 |
| Molar mass | 439.46 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Althiomycin (matamycin) is a thiazole antibiotic, effective against Gram-positive and Gram-negative bacteria. The name matamycin is from "Mata Hari" and the suffix -mycin.
Isolated from Streptomyces matensis, the compound was first described by Margalith et al. in 1959. It acts a protein synthesis inhibitor and its site of action is the 50S subunit of the bacterial ribosome.
References
- Aronson J (October 1999). "That's show business". BMJ (Clinical Research Ed.). 319 (7215): 972. doi:10.1136/bmj.319.7215.972. PMC 1116803. PMID 10514162.
- ^ Pestka S (1975). "Althiomycin". In Corcoran JW, Hahn FE, Snell JF, Arora KL (eds.). Mechanism of Action of Antimicrobial and Antitumor Agents. Antibiotics. Berlin Heidelberg: Springer-Verlag. pp. 323–6. doi:10.1007/978-3-642-46304-4_21. ISBN 978-3-642-46304-4.