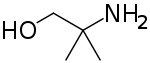
| |
| Names | |
|---|---|
| Preferred IUPAC name 2-Amino-2-methylpropan-1-ol | |
| Other names
Isobutanol-2-amine Aminoisobutanol 2-Amino-2-methyl-1-propanol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.004.282 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H11NO |
| Molar mass | 89.138 g·mol |
| Density | 0.934 g/cm |
| Melting point | 30–31 °C (86–88 °F; 303–304 K) |
| Boiling point | 165.5 °C (329.9 °F; 438.6 K) |
| Solubility in water | Miscible |
| Solubility in alcohols | Soluble |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H412 |
| Precautionary statements | P264, P264+P265, P273, P280, P302+P352, P305+P351+P338, P321, P332+P317, P337+P317, P362+P364, P501 |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Aminomethyl propanol (AMP) is an organic compound with the formula H2NC(CH3)2CH2OH. It is colorless liquid that is classified as an alkanolamine. It is a useful buffer and a precursor to numerous other organic compounds.
Aminomethyl propanol is typically sold as a solution of the material in water, for which different concentrations are available.
Synthesis
Aminomethyl propanol can be produced by the hydrogenation of 2-aminoisobutyric acid or its esters.
Properties
Aminomethyl propanol is soluble in water and about the same density as water.
Uses
Aminomethyl propanol is used for the preparation of buffer solutions. It is a component of the drugs ambuphylline and pamabrom. It is also used in cosmetics.
It is a precursor to oxazolines via its reaction with acyl chlorides. Via sulfation of the alcohol, the compound is also a precursor to 2,2-dimethylaziridine.
Aminomethyl propanol is used as an intermediate the synthesis of fepradinol, isobucaine, and radafaxine.
References
- "2-Amino-2-methyl-1-propanol". pubchem.ncbi.nlm.nih.gov.
- ^ "Aminomethyl-propanol". Cosmetics Info. Archived from the original on 14 August 2014. Retrieved 14 August 2014.
- ^ "2-Amino-2-methyl-1-propanol". Chemical Book. Retrieved 14 August 2014.
- Bougie, Francis; Iliuta, Maria (2012-02-14). "Sterically Hindered Amine-Based Absorbents for the Removal of CO2 from Gas Streams". J Chem Eng Data. 57 (3): 635–669. doi:10.1021/je200731v.
- Albert I. Meyers; Mark E. Flanagan (1993). "2,2'-Dimethoxy-6-Formylbiphenyl". Org. Synth. 71: 107. doi:10.15227/orgsyn.071.0107.
- Kenneth N. Campbell; Armiger H. Sommers; Barbara K. Campbell; Lee Irvin Smith; Oliver H. Emerson; D. E. Pearson; J. F. Baxter; K. N. Carter (1947). "Tert-butylamine". Org. Synth. 27: 12. doi:10.15227/orgsyn.027.0012.