| |
| Names | |
|---|---|
| IUPAC name Antimony(III) sulfate | |
| Other names
Antimonous sulfate Antimony trisulfate Diantimony trisulfate Diantimony tris(sulphate) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.028.370 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
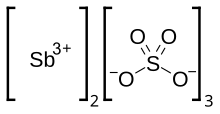
| Chemical formula | Sb2(SO4)3 |
| Molar mass | 531.7078 g/mol |
| Density | 3.94 g/cm |
| Solubility in water | Hydrolysis |
| Structure | |
| Crystal structure | monoclinic |
| Space group | P21/c |
| Lattice constant | a = 13.12 Å, b = 4.75 Å, c = 17.55 Åα = 90°, β = 126.3°, γ = 90° |
| Lattice volume (V) | 881 Å |
| Hazards | |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA 0.5 mg/m (as Sb) |
| REL (Recommended) | TWA 0.5 mg/m (as Sb) |
| Safety data sheet (SDS) | MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Antimony sulfate, Sb2(SO4)3, is a hygroscopic salt formed by reacting antimony or its compounds with hot sulfuric acid. It is used in doping of semiconductors and in the production of explosives and fireworks.
Structure
Antimony(III) sulfate consists of interconnected SbO6 octahedra, which the corners are bonded to the sulfate ion.
Production
Antimony(III) sulfate was first produced in 1827 by the reaction of antimony(III) oxide and 18 molar sulfuric acid at 200 °C:
- Sb2O3 + 3 H2SO4 → Sb2(SO4)3 + 3 H2O
The concentration of the sulfuric acid is important, as a lower concentration will produce basic antimony oxides, while a higher concentration will produce antimony(III) pyrosulfate. The reaction of elemental antimony and 18 M sulfuric acid will also produce antimony(III) sulfate:
- 2 Sb + 6 H2SO4 → Sb2(SO4)3 + 3 SO2 + 6 H2O
Chemical properties
Antimony sulfate is deliquescent, hydrolyzing in moist air and water, producing various basic antimony oxides and antimony(III) oxide. It is soluble in acids.
Uses
Owing to its solubility, antimony sulfate has uses in the doping of semiconductors. It is also used for coating anodes in electrolysis and in the production of explosives and fireworks.
Safety
Antimony(III) sulfate causes irritation to the skin and mucous membranes.
Natural occurrence
Natural analogue of the exact compound is yet unknown. However, basic hydrated Sb sulfates are known as the minerals klebelsbergite and coquandite.
References
- ^ R. Mercier; J. Douglade; J. Bernard (1976). "Structure cristalline de Sb2O3.3SO3". Acta Crystallographica Section B (in French). 32 (10): 2787–2791. doi:10.1107/S0567740876008881.
- Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. p. 4.64. ISBN 0-8493-0486-5.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- ^ Herbst, Karl Albert et al. (1985) Antimony and antimony compounds in Ullmann's Encyclopedia of Industrial Chemistry 5th ed., vol. A3, p. 70. ISBN 3-527-20103-3.
- Nicholas C. Norman (31 December 1997). Chemistry of arsenic, antimony, and bismuth. Springer. pp. 193–. ISBN 978-0-7514-0389-3.
- Method of forming phase change layer, method of manufacturing a storage node using the same, and method of manufacturing phase change memory device using the same – Samsung Electronics Co., Ltd. Freepatentsonline.com (2007-01-02). Retrieved on 2011-12-23.
- Antimony(III) Sulfate Material Safety Data Sheet Archived 2012-04-26 at the Wayback Machine. Prochemonline.
- "Klebelsbergite".
- ^ "List of Minerals". 21 March 2011.
- "Coquandite".
| Antimony compounds | |||
|---|---|---|---|
| Antimonides | |||
| Sb(III) |
| ||
| Sb(III,V) | |||
| Sb(V) |
| ||