| |
| Names | |
|---|---|
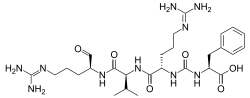
| IUPAC name N-{carbamoyl}-N-(diaminomethylidene)-L-ornithyl-N-{(2S)-5--1-oxopentan-2-yl}-L-valinamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C27H44N10O6 |
| Molar mass | 604.713 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain. It was discovered in 1972 and was the first natural peptide found that contained an ureylene group. Antipain can aid in prevention of coagulation in blood. It is an inhibitor of serine and cysteine proteases.
It has been crystallized in complexes with carboxypeptidase, which is obtained from wheat, and Leishmania major oligopeptidase B. In both cases, the backbone carbonyl of the terminal arginine of antipain forms a covalent bond to the active site serine in the protease.
A study was performed for information on the effect of antipain on the quality of post-thawed ram semen. The results from this experiment concluded that antipain aided in the quality of ram semen by maintaining the sperm mobility. Antipain includes the function to inhibit a degrading enzyme, called plasmin, permitting this substance to be able to improve the resistance of membrane disruption by freezing temperatures.
Antipain Y, a similar chemical compound that is an analog of antipain, which was isolated from a species of Streptomyces, inhibits the release of neurotransmitters in rat dorsal root ganglion neurons.
References
- Suda H, Aoyagi T, Hamada M, Takeuchi T, Umezawa H (April 1972). "Antipain, a new protease inhibitor isolated from actinomycetes". The Journal of Antibiotics. 25 (4): 263–266. doi:10.7164/antibiotics.25.263. PMID 4559651.
- Umezawa S, Tatsuta K, Fujimoto K, Tsuchiya T, Umezawa H (April 1972). "Structure of antipain, a new Sakaguchi-positive product of streptomyces". The Journal of Antibiotics. 25 (4): 267–270. doi:10.7164/antibiotics.25.267. PMID 5052959.
- Lackie J (2012). A Dictionary of Biomedicine. Oxford University Press. ISBN 9780199549351.
- PDB ENTRY 1bcr Bullock TL, Breddam K, Remington SJ (February 1996). "Peptide aldehyde complexes with wheat serine carboxypeptidase II: implications for the catalytic mechanism and substrate specificity". Journal of Molecular Biology. 255 (5): 714–725. doi:10.1006/jmbi.1996.0058. PMID 8636973.
- PDB ENTRY 2xe4 McLuskey K, Paterson NG, Bland ND, Isaacs NW, Mottram JC (December 2010). "Crystal structure of Leishmania major oligopeptidase B gives insight into the enzymatic properties of a trypanosomatid virulence factor". The Journal of Biological Chemistry. 285 (50): 39249–39259. doi:10.1074/jbc.M110.156679. PMC 2998157. PMID 20926390.
- ^ Akhtarshenas, Bahareh; Karami Shabankareh, Hamed; Hajarian, Hadi; Bucak, Mustafa Numan; Abdolmohammadi, Ali Reza; Dashtizad, Mojtaba (2018). "The protease inhibitor antipain has a beneficial synergistic effect with trehalose for ram semen cryopreservation". Reproduction in Domestic Animals. 53 (6): 1359–1366. doi:10.1111/rda.13253. PMID 30011087. S2CID 51629940.
- Nakae K, Kojima F, Sawa R, Kubota Y, Igarashi M, Kinoshita N, et al. (January 2010). "Antipain Y, a new antipain analog that inhibits neurotransmitter release from rat dorsal root ganglion neurons". The Journal of Antibiotics. 63 (1): 41–44. doi:10.1038/ja.2009.109. PMID 19911027.