| |
| Names | |
|---|---|
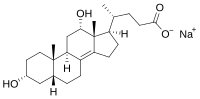
| IUPAC name 3α,12α-Dihydroxy-5β-chol-8(14)-en-24-oic acid | |
| Systematic IUPAC name (4R)-4-phenanthren-1-yl]pentanoic acid | |
| Other names 3α,12α-Dihydroxy-5β,8(14)-cholen-24-oic acid; 5β,8(14)-Cholen-24-oic acid-3α,12α-diol | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C24H38O4 |
| Molar mass | 390.564 g·mol |
| Melting point | 175 to 176 °C (347 to 349 °F; 448 to 449 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Apocholic acid is an unsaturated bile acid first characterized in the 1920s. It has questionable carcinogenic activity as experimentally, sarcomas were induced in mice with injection of deoxycholic acid.
The salts and esters of apocholic acid are known as apocholates.
See also
References
- |ALDRICH&N5=SEARCH_CONCAT_PNO|BRAND_KEY&F=SPEC Apocholic acid at Sigma-Aldrich
- Boedecker, F.; Volk, H. (1922). "Unsaturated bile acids. III. Relations of apocholic acid, dihydroxycholenic acid (m. 260) and cholic acid to desoxycholic acid". Berichte der Deutschen Chemischen Gesellschaft B. 55: 2302–2309. doi:10.1002/cber.19220550810.
- Lacassagne, A (June 10, 1961). "Carcinogenic activity of apocholic acid". Nature. 190 (4780): 1007–8. Bibcode:1961Natur.190.1007L. doi:10.1038/1901007a0. PMID 13831121. S2CID 4175440.