| |

| |
| Names | |
|---|---|
| Systematic IUPAC name Arsonium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| Gmelin Reference | 322800 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
|
SMILES
| |
| Properties | |
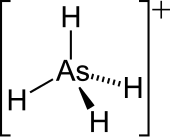
| Chemical formula | AsH 4 |
| Molar mass | 78.954 g·mol |
| Conjugate base | Arsine |
| Structure | |
| Molecular shape | Tetrahedral |
| Related compounds | |
| Related compounds | ammonium phosphonium |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |

The arsonium cation is a positively charged polyatomic ion with the chemical formula AsH
4. An arsonium salt is a salt containing either the arsonium (AsH
4) cation, such as arsonium bromide (AsH
4Br
) and arsonium iodide (AsH
4I
), which can be synthesized by reacting arsine with hydrogen bromide or hydrogen iodide. Or more commonly, as organic derivative such as the quaternary arsonium salts Ph
4As
Cl
(CAS: 123334-18-9 , hydrate form) and the zwitterionic compound arsenobetaine.
References
- Muñoz-Hernández, M. Á. (2006). Arsenic: Inorganic Chemistry. Encyclopedia of Inorganic Chemistry. pp 4. DOI: 10.1002/0470862106.ia013
This article about chemical compounds is a stub. You can help Misplaced Pages by expanding it. |