 | |
| Clinical data | |
|---|---|
| Other names | 17-ethyl-1,14-dihydroxy-12--23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclooctacos-18-ene-2,3,10,16-tetraone |
| ATC code |
|
| Identifiers | |
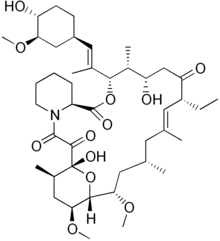
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.108.430 |
| Chemical and physical data | |
| Formula | C43H69NO12 |
| Molar mass | 792.020 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Ascomycin, also called Immunomycin, FR-900520, FK520, is an ethyl analog of tacrolimus (FK506) with strong immunosuppressant properties. It has been researched for the treatment of autoimmune diseases and skin diseases, and to prevent rejection after an organ transplant.
Ascomycin acts by binding to immunophilins, especially macrophilin-12. It appears that Ascomycin inhibits the production of Th1 (interferon- and IL-2) and Th2 (IL-4 and IL-10) cytokines. Additionally, ascomycin preferentially inhibits the activation of mast cells, an important cellular component of the atopic response. Ascomycin produces a more selective immunomodulatory effect in that it inhibits the elicitation phase of allergic contact dermatitis but does not impair the primary immune response when administered systemically.
Ascomycin is produced by the fermentation of certain strains of Streptomyces hygroscopicus.
In fiction
Ascomycin is also the name of a fictional "antiagathic" (anti-aging) drug in James Blish's future history Cities in Flight. and in its component novel They Shall Have Stars.
Related compounds
References
- Andexer JN, Kendrew SG, Nur-e-Alam M, Lazos O, Foster TA, Zimmermann AS, et al. (March 2011). "Biosynthesis of the immunosuppressants FK506, FK520, and rapamycin involves a previously undescribed family of enzymes acting on chorismate". Proceedings of the National Academy of Sciences of the United States of America. 108 (12): 4776–4781. doi:10.1073/pnas.1015773108. PMC 3064383. PMID 21383123.
- Paul C, Graeber M, Stuetz A (January 2000). "Ascomycins: promising agents for the treatment of inflammatory skin diseases". Expert Opinion on Investigational Drugs. 9 (1): 69–77. doi:10.1517/13543784.9.1.69. PMID 11060661. S2CID 19730971.
- Yu Z, Lv H, Wu Y, Wei T, Yang S, Ju D, Chen S (December 2019). "Enhancement of FK520 production in Streptomyces hygroscopicus by combining traditional mutagenesis with metabolic engineering". Applied Microbiology and Biotechnology. 103 (23–24): 9593–9606. doi:10.1007/s00253-019-10192-8. PMID 31713669. S2CID 207955563.
- "Anti-agathic drugs". Technovelgy.com. Retrieved 15 June 2022.
Further reading
- Griffiths CE (April 2001). "Ascomycin: an advance in the management of atopic dermatitis". The British Journal of Dermatology. 144 (4): 679–681. doi:10.1046/j.1365-2133.2001.144004679.x. PMID 11298524. S2CID 46503687.
- Kawai M, Lane BC, Hsieh GC, Mollison KW, Carter GW, Luly JR (January 1993). "Structure-activity profiles of macrolactam immunosuppressant FK-506 analogues". FEBS Letters. 316 (2): 107–113. doi:10.1016/0014-5793(93)81196-7. PMID 7678400. S2CID 24298979.
- Zuberbier T, Chong SU, Grunow K, Guhl S, Welker P, Grassberger M, Henz BM (August 2001). "The ascomycin macrolactam pimecrolimus (Elidel, SDZ ASM 981) is a potent inhibitor of mediator release from human dermal mast cells and peripheral blood basophils". The Journal of Allergy and Clinical Immunology. 108 (2): 275–280. doi:10.1067/mai.2001.116865. PMID 11496246.
- Mollison KW, Fey TA, Krause RA, Thomas VA, Mehta AP, Luly JR (June 1993). "Comparison of FK-506, rapamycin, ascomycin, and cyclosporine in mouse models of host-versus-graft disease and heterotopic heart transplantation". Annals of the New York Academy of Sciences. 685: 55–57. doi:10.1111/j.1749-6632.1993.tb35851.x. PMID 7689812. S2CID 28990806.
- Paul C, Graeber M, Stuetz A (January 2000). "Ascomycins: promising agents for the treatment of inflammatory skin diseases". Expert Opinion on Investigational Drugs. 9 (1): 69–77. doi:10.1517/13543784.9.1.69. PMID 11060661. S2CID 19730971.
External links
- Exciting New Eczema Treatment Expected This Year By Jane Schwanke, WebMD Medical News March 17, 2000 (San Francisco)