| |
| Names | |
|---|---|
| IUPAC name (R-(R*,R*))-N-(2-((2-Amino-2-carboxyethyl)amino)-2-carboxyethyl)-L-aspartic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H17N3O8 |
| Molar mass | 307.257 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Aspergillomarasmine A is an polyamino acid naturally produced by the mold Aspergillus versicolor. The substance has been reported to inhibit two antibiotic resistance carbapenemase proteins in bacteria, New Delhi metallo-beta-lactamase 1 (NDM-1) and Verona integron-encoded metallo-beta-lactamase (VIM-2), and make those antibiotic-resistant bacteria susceptible to antibiotics. Aspergillomarasmine A is toxic to leaves of barley and other plants, being termed as "Toxin C" when produced by Pyrenophora teres.
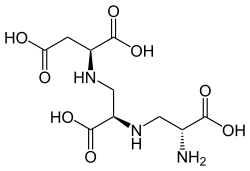
The molecule is a tetracarboxylic acid with four -COOH groups. One section of the molecule is the amino acid aspartic acid. This has two alanine molecules attached by substituting a hydrogen on the methyl group with a link to the amine group. Aspergillomarasmine B differs in that the last alanine is replaced by glycine.
The crystalline substance was first isolated in 1956, but its name was given until 1965.
In addition to Aspergillus versicolor, aspergillomarasmine A is also produced by the ascomycete Pyrenophora teres where it acts as a toxin in the barley net-spot blotch disease. In P. teres, a biosynthetic precursor of aspergillomarasmine A, L,L-N-(2-amino-2-carboxyethyl)-aspartic acid has also been isolated and found to contribute to the phytotoxic properties of this microbe. This precursor, aspergillomarasmine A itself, and a lactam form (anhydroaspergillomarasmine A) are together termed the marasmines.
Other producers of aspergillomarasmine A include Aspergillus flavus, Aspergillus oryzae, Colletotrichum gloeosporioides, and Fusarium oxysporum.
In mice the LD50 toxic dose of aspergillomarasmine A is 159.8 mg/kg.
Properties
Aspergillomarasmine A takes the form of colourless crystals. The chemical is insoluble in common organic solvents, but can dissolve in water under either basic or strongly acidic conditions.
Anhydroaspergillomarasmine A, a lactam of aspergillomarasmine A, chemically called , can also be found in Pyrenophora teres. The relative amount of these two toxins is dependent upon the pH of the growth medium, with lower pH favouring the lactam form. The lactam can be hydrolyzed to aspergillomarasmine A by treating it with trifluoroacetic acid.
Aspergillomarasmine A functions as a chelating agent, sequestering Fe ions. It can inhibit endothelin converting enzymes even in the live rat, probably by chelating metals required by metalloproteases.
When heated, aspergillomarasmine A decomposes between 225° and 236 °C. Hydrolysis produces L-aspartic acid and racemic 2,3-diaminopropionic acid. Even though the precursor component is chiral, 2,3-diaminopropionic acid easily racemizes in acid.
Aspergillomarasmine A has D at pH 7 of -48°.
With nitrous acid aspergillomarasmine A is deaminated, and isoserine with aspartic acid is formed.
Titration reveals changes in ionisation at pK 3.5 and 4.5 due to carboxylic acid groups, and pK 9.5 and 10 due to amino groups.
Treatment with ninhydrin shows a purple colour.
References
- King, Andrew M.; Sarah A. Reid-Yu; Wenliang Wang; Dustin T. King; Gianfranco De Pascale; Natalie C. Strynadka; Timothy R. Walsh; Brian K. Coombes; Gerard D. Wright (2014). "Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance". Nature. 510 (7506): 503–506. Bibcode:2014Natur.510..503K. doi:10.1038/nature13445. ISSN 0028-0836. PMC 4981499. PMID 24965651.
- ^ Weiergang, I.; H.J. Lyngs Jørgensen; I.M. Møller; P. Friis; V. Smedegaard-Petersen (2002). "Optimization of in vitro growth conditions of Pyrenophora teres for production of the phytotoxin aspergillomarasmine A". Physiological and Molecular Plant Pathology. 60 (3): 131–140. doi:10.1006/pmpp.2002.0383. ISSN 0885-5765.
- ^ Haenni, A. L.; M. Robert; W. Vetter; L. Roux; M. Barbier; E. Lederer (1965). "Structure chimique des aspergillomarasmines A et B" [Chemical structure of aspergellomarasmines A and B]. Helvetica Chimica Acta (in French). 48 (4): 729–750. doi:10.1002/hlca.19650480409. ISSN 0018-019X. PMID 14321962.
- Friis, P; Olsen C.E.; Møller B.L. (15 July 1991). "Toxin production in Pyrenophora teres, the ascomycete causing the net-spot blotch disease of barley (Hordeum vulgare L.)". The Journal of Biological Chemistry. 266 (20): 13329–13335. doi:10.1016/S0021-9258(18)98843-5. PMID 2071605.
- Wagman, G.H.; Cooper, R. (1988-12-01). Natural Products Isolation: Separation Methods for Antimicrobials, Antivirals and Enzyme Inhibitors. Elsevier. p. 499. ISBN 9780080858487. Retrieved 27 June 2014.
- Matsuura, Akihiro; Hiroshi Okumura; Rieko Asakura; Naoki Ashizawa; Mayumi Takahashi; Fujio Kobayashi; Nami Ashikawa; Koshi Arai (1993). "Pharmacological profiles of aspergillomarasmines as endothelin converting enzyme inhibitors". The Japanese Journal of Pharmacology. 63 (2): 187–193. doi:10.1254/jjp.63.187. PMID 8283829.
- Barbier, M. (1987). "Remarks on the biological activity of aspergillomarasmine A Fe chelate and other iron transporting phytotoxins with reference to their role in the photodegradation of aromatic amino-acids in infected plant leaves". Journal of Phytopathology. 120 (4): 365–368. doi:10.1111/j.1439-0434.1987.tb00500.x. ISSN 0931-1785.
- Huggins, John P.; Pelton, John T. (1996-12-23). Endothelins in Biology and Medicine. CRC Press. p. 121. ISBN 9780849369759. Retrieved 27 June 2014.