| |
| Names | |
|---|---|
| IUPAC name 12,14a,3-(Epoxymethyno)-2H-1-benzoxacyclododecin-2,4,8(5H,10aH)-trione, 6,7,11,12,13,14-hexahydro-11-hydroxy-5,7,13-trimethyl-, (5R,7S,9E,10aR,11R,12R,13R,14aR) | |
| Other names Atrop-abyssomicin C | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C19H22O6 |
| Molar mass | 346.38 g/mol |
| Density | 1.34±0.1 g/cm3 (Predicted) |
| Melting point | 180 °C (decomp) |
| Boiling point | 597.5±50.0 °C (Predicted) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
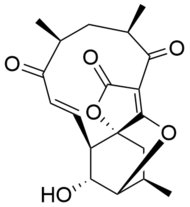
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin. In 2006, the Nicolaou group discovered atrop-abyssomicin C while working on the total synthesis of abyssomicin C. Then in 2007, Süssmuth and co-workers isolated atrop-abyssomicin C from Verrucosispora maris AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that atrop-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB (4-amino-4-deoxychorismate synthase), thereby depleting the biosynthesis of p-aminobenzoate.
Structure
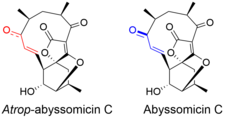
Atrop-abyssomicin C has a complex, yet intriguing structural topography. The compound contains an oxabicyclooctane system fused to the tetronate moiety. The 11-membered macrocyclic ring carries an α,β-unsaturated ketone that was proposed to be the reactive center. Despite being a strained macrocycle, there exist an atropisomer, abyssomicin C. The atropisomerism arise due to a structural deviation in the α,β-unsaturated ketone region of the molecule. The orientation of the carbonyl in atrop-abyssomicin C is cisoid, whereas the conformation in abyssomicin C is transoid. The enone moiety of atrop-abyssomicin C has a higher degree of the conjugation, which makes it a more active Michael acceptor.
Biosynthesis
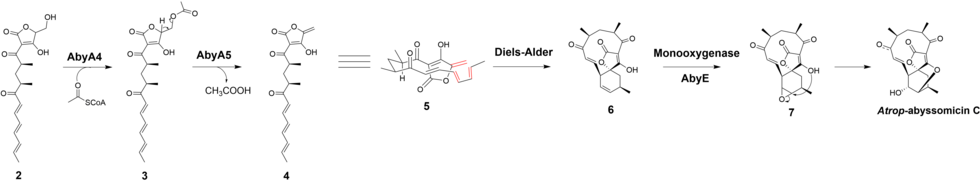
The biosynthesis of atrop-abyssomicin C begins with the synthesis of a linear polyketide chain in a PKS I system that consist of one loading and six extension modules. The polyketide chain is made from five acetates, two propionates, and the glycolytic pathway metabolite. D-1,3-bisphosphoglycerate, the glycolytic metabolite, is transferred to AbyA3 (an acyl-carrier protein) by AbyA2 to generate the glyceryl-ACP. AbyA1 facilitates the attachment of the glyceryl-ACP to the polyketide chain and the detachment of the polyketide from the polyketide synthase to form intermediate 2.

Based on the observation made for the biosynthesis of agglomerin, it has been proposed that AbyA4 acetylates intermediate 2 and AbyA5 catalyzes the elimination of acetic acid to form the exocyclic double bond in intermediate 4. An intramolecular Diels-Alder was proposed to take place between the exocyclic olefin and the conjugated diene at the tail end of the polyketide to form the macrocyclic ring. It has been reported that the previously unidentified Abycyc gene could code for an enzyme that carries out the Diels-Alder cycloaddition. Following the Diels-Alder reaction, an epoxide ring is formed and then opened by the tetronate hydroxyl group to form atrop-abyssomicin C. It has been postulated that the AbyE monooxygenase catalyzes epoxide formation.
References
- ^ Kanchanabanca C, Tao, W., Hong, H., Liu, Y., Hanh, F., Samborskyy, M., Deng, Z., Sun, Y., Leadlay, P.F. (2013). "Unusual acetylation-elimination in the formation of tetronate antibiotics". Angewandte Chemie International Edition. 52 (22): 5785–8. doi:10.1002/anie.201301680. PMID 23606658.
- Nicolaou K, Harrison, S.T. (2006). "Total Synthesis of Abyssomicin C and atrop-Abyssomicin C**". Angewandte Chemie International Edition. 45 (20): 3256–60. doi:10.1002/anie.200601116. PMID 16634106.
- Keller S, Nicholson, G., Drahl, C., Sorensen, E., Fiedler, H.P., Süssmuth, R.D. (2007). "Abyssomicins G and H and atrop-abyssomicin C from the marine Verrucosispora strain AB-18-032". Journal of Antibiotics. 60 (6): 391–4. doi:10.1038/ja.2007.54. PMID 17617698.
- Keller S, Schadt HS, Ortel I, Süssmuth RD (2007). "Action of atrop-abyssomicin C as an inhibitor of 4-amino-4-deoxychorismate synthase PabB". Angew. Chem. Int. Ed. Engl. 46 (43): 8284–6. doi:10.1002/anie.200701836. PMID 17886307.
- Nicolaou K, Harrison, S.T., Chen, J.S. (2009). "Discoveries from the Abyss: The Abyssomicins and Their Total Synthesis". Synthesis. 2009 (1): 33–42. doi:10.1055/s-0028-1083259. PMC 2677807. PMID 20047014.
- Nicolaou K, Harrison, S.T. (2007). "Total synthesis of abyssomicin C, atrop-abyssomicin C, and abyssomicin D: implications for natural origins of atrop-abyssomicin C". Journal of the American Chemical Society. 129 (2): 429–40. doi:10.1021/ja067083p. PMID 17212423.
- ^ Savic V (2013). "Chapter 5 – Abyssomicins: Isolation, Properties, and Synthesis". Studies in Natural Products Chemistry. 40: 133–172. doi:10.1016/B978-0-444-59603-1.00005-9.
- ^ Gottardi E, Krawczyk, J., von Suchodoletz, H., Schadt, S., Mühlenweg, A., Uguru, G., Süssmuth, R.D. (2011). "Abyssomicin Biosynthesis: Formation of an Unusual Polyketide, Antibiotic-Feeding Studies and Genetic Analysis". ChemBioChem. 12 (9): 1401–1410. doi:10.1002/cbic.201100172. PMC 3625739. PMID 21656887.
- Vieweg L, Reichau, S., Schobert, R, Leadlay, P.F., Süssmuth, R. (2014). "Recent advances in the field of bioactive tetronates". Natural Product Reports. 31 (11): 1554–1584. doi:10.1039/c4np00015c. PMID 24965099.
- Hashimoto T, Hashimoto, J., Teruya, K., Hirano, T., Shin-ya, K., Ikeda, H., Liu, H.W., Nishiyama, M., Kuzuyama T. (2015). "Biosynthesis of versipelostatin: identification of an enzyme-catalyzed [4+2]-cycloaddition required for macrocyclization of spirotetronate-containing polyketides". Journal of the American Chemical Society. 137 (2): 572–5. doi:10.1021/ja510711x. PMC 4308742. PMID 25551461.