The correct title of this article is Benzofluoranthene. The substitution of any brackets is due to technical restrictions.
| |
| Names | |
|---|---|
| Preferred IUPAC name Benzofluoranthene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.005.374 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3077 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H12 |
| Molar mass | 252.3093 |
| Appearance | solid |
| Density | 1.286 g/cm3 |
| Melting point | 165 °C (329 °F; 438 K) |
| Hazards | |
| Flash point | 228.6 °C (443.5 °F; 501.8 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
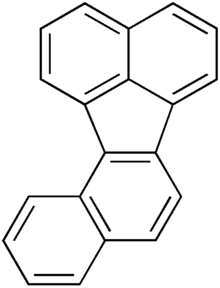
Benzofluoranthene (BjF) is an organic compound with the chemical formula C20H12. Classified as a polycyclic aromatic hydrocarbon (PAH), it is a colourless solid that is poorly soluble in most solvents. Impure samples can appear off white. Closely related isomeric compounds include benzofluoranthene (BaF), benzofluoranthene (BbF), benzofluoranthene (BeF), and benzofluoranthene (BkF). BjF is present in fossil fuels and is released during incomplete combustion of organic matter. It has been traced in the smoke of cigarettes, exhaust from gasoline engines, emissions from the combustion of various types of coal and emissions from oil heating, as well as an impurity in some oils such as soybean oil.
Structure and synthesis
BjF consists of two naphthalene-like structures which are fused by a cyclopentane structure. This cyclopentane is not included in the aromaticity of the molecule. BjF can be obtained when either 2-(1-chloroethenyl)benzophenanthrene or 6-(1-chloroethenyl)chrysene is treated by flash vacuum thermolysis (FVT) at high temperatures (above 900 °C) followed by ring rearrangements (ring contraction/expansion) to selectively yield BjF. Benzofluoranthene may also be converted via similar processes to BjF by FVT at temperatures of at least 1100 °C (6% yield) or at least 1200 °C (11% yield) with 38% mass recovery.
Reactivity
BjF can be functionalized by means of electrophilic aromatic substitution. In the body it is metabolized into phenols (3,4,6 or 10 hydroxy), dihydrodiols (4,5 and 9,10) and 4,5-dione (fig. 1).

Mechanism of action
BjF is categorized by the IARC as possibly carcinogenic to human beings, like many other PAHs, on the basis of sufficient evidence in animals. For example, BjF is active as a tumor initiator on mouse skin and is carcinogenic in both mouse skin and in rat lungs. Recently, BjF was also found to induce tumors in newborn mouse lung and liver. The mechanism of actions of BjF is similar to other PAHs. The diolepoxide mechanism involves formation of stable and unstable DNA adducts, mainly at G and A, which can lead to mutations in proto-oncogenes (RAS) and tumour-suppressor genes (P53). Many polycyclic aromatic hydrocarbon diolepoxides and their precursor diols and epoxides are tumorigenic in animals. The radical cation mechanism involves generation of unstable adducts at G and A, leading to apurinic sites and mutations in HRAS. Orthoquinone formation could lead to stable and unstable DNA adducts and generation of reactive oxygen species, inducing mutations in P53.
Toxicity
PAHs
One of the earliest connection between PAHs, combustion, and cancer was established by Cook and co-workers with the isolation of the carcinogen benzopyrene from coal tar extract. Benzopyrene now has been well characterized in toxicology reports and is a known potent carcinogen. Benzopyrene requires metabolic activation to become, ultimately, BPDE ((±)-anti-7β,8α-dihydroxy-9α,10α-epoxy-7,8,9,10-tetrahydrobenzopyrene) which binds to the DNA to form a covalent trans adducts at the N2 position of guanine. Hereafter binding to DNA at cancer hotspots, especially in the P53 tumour suppressor gene at codons: 157, 248 and 273 (figure 3), it has the possibility of inducing lung cancer. Structural similarity of PAHs contributes to the similarity in metabolism, biotransformation and toxicology. Benzopyrene has been extensively reviewed and is used as a model for the toxicology and metabolism of other PAHs.
Benzofluoranthene
Specific studies on BjF showed that it exhibits mutagenic toxicity in S. typhimurium TA98 and TA1000 under the presence of microsomal activation. BjF can form DNA-adducts, covalently binding of chemicals to DNA can result in strand breaks and DNA damage, which ultimately leads to mutations. In mice studies BjF induced tumorigenic activity on the skin, lung adenomas and liver adenomas/hepatomas. Lung implantation of BjF also induced lung epidermoid carcinomas in 3-month-old female rats. Tail vein injection of BjF also causes covalently binding to mouse hemoglobin and serum proteins, with binding to serum proteins being 10-fold higher than to hemoglobin.
References
- Author unknown (23 June 2005) "Benzofluoranthene". TOXNET http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~ZHcJmU:1 (last consulted on: 19 March 2015)
- Author unknown (date unknown) "Benzofluoranthene" Sigma-Aldrich (last consulted on: 19 March 2015)
- M. Sarobe et al. (1 January 1997) "High temperature gas phase syntheses of C20H12 cyclopenta-fused polycyclic aromatic hydrocarbons: benzacephenanthrylene and benzacephenanthrylene and their selective rearrangement to benzofluoranthene" Journal of the Chemical Society, Perkin Trans. 2
- M. Sarobe et al. (1999) "Flash Vacuum Thermolysis of Acenaphthoacenaphthylene, Fluoranthene, Benzo- and Benzofluoranthene 2 Homolytic Scission of Carbon2Carbon Single Bonds of Internally Fused Cyclopenta Moieties at T ≥ 1100°C" European Journal of Organic Chemistry
- E.H. Weyand et al. (1993) "Detection of the Major DNA Adducts of Benzofluoranthene in Mouse Skin: Nonclassical Dihydrodiol Epoxides" Chemical Research in Toxicology 6
- K.Straif et al. (December 2005) "Carcinogenicity of polycyclic aromatic hydrocarbons" THE LANCET Oncology, vol.6, issue 12
- J.E. Rice et al. (1 December 1987) "Identification of Tumorigenic Metabolites of Benzofluoranthene Formed in Vivo in Mouse Skin" Cancer Research 47, 6166-6170
- K.Straif et al. (December 2005) "Carcinogenicity of polycyclic aromatic hydrocarbons" The Lancet Oncology, vol.6, issue 12
- Cook, James Wilfred, C. L. Hewett, and I. Hieger. "106. The isolation of a cancer-producing hydrocarbon from coal tar. Parts I, II, and III." Journal of the Chemical Society (1933): 395-405.
- Denissenko, Mikhail F., et al. "Preferential formation of benzopyrene adducts at lung cancer mutational hotspots in P53." Science 274.5286 (1996): 430-432.
- Singer, B., and D. Grunberger. "Metabolic activation of carcinogens and mutagens." Molecular Biology of Mutagens and Carcinogens. Springer US, 1983. 97-141. ISBN 978-1-4613-3772-0
- Denissenko, Mikhail F., et al. "Preferential formation of benzopyrene adducts at lung cancer mutational hotspots in P53." Science 274.5286 (1996): 430-432.
- Agency for Toxic Substances and Disease Registry (ATSDR). (1995) "Toxicological profile for Polycyclic Aromatic Hydrocarbons (PAHs)" Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
- E.J. Lavoie et al. (December 1980) "Identification of Mutagenic Dihydrodiols as Metabolites of Benzofluoranthene and Benzofluoranthene" Cancer Research 40, 4528-4532
- Agency for Toxic Substances and Disease Registry (ATSDR). (1995) "Toxicological profile for Polycyclic Aromatic Hydrocarbons (PAHs)" Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
- LaVoie, Edmond J., et al. "Tumour initiating activity of dihydrodiols of benzofluoranthene, benzofluoranthene, and benzofluoranthene." Carcinogenesis 3.1 (1982): 49-52.
- Habs, M., D. Schmähl, and J. Misfeld. "Local carcinogenicity of some environmentally relevant polycyclic aromatic hydrocarbons after lifelong topical application to mouse skin." Archiv für Geschwulstforschung 50.3 (1979): 266-274.
- Wynder, Ernest L., and Dietrich Hoffmann. "The carcinogenicity of benzofluoranthenes." Cancer 12.6 (1959): 1194-1199.
- Weyand, E. H., et al. "Effect of fluorine substitution on benzofluoranthene genotoxicity." Chemico-Biological Interactions 84.1 (1992): 37-53
- Deutsch-Wenzel, Reintraud P., et al. "Experimental studies in rat lungs on the carcinogenicity and dose-response relationships of eight frequently occurring environmental polycyclic aromatic hydrocarbons." Journal of the National Cancer Institute 71.3 (1983): 539-544
- Agency for Toxic Substances and Disease Registry (ATSDR). (1995) "Toxicological profile for Polycyclic Aromatic Hydrocarbons (PAHs)" Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
| Polycyclic aromatic hydrocarbons | |
|---|---|
| 2 rings | |
| 3 rings | |
| 4 rings | |
| 5 rings | |
| 6 rings | |
| 7+ rings | |
| General classes | |