| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
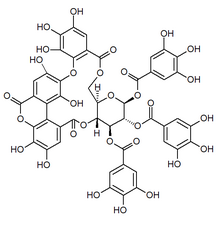
| Chemical formula | C48H32O30 |
| Molar mass | 1088.754 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Bicornin is an ellagitannin found in plants of the order Myrtales, including Trapa bicornis (water caltrop) and Syzygium aromaticum (clove).
The molecule contains a luteic acid group.
References
- Yoshida T, Yazaki K, Memon M.U, Maruyama I, Kurokawa K and Okuda T (1989). "Bicornin, a new hydrolyzable tannin from Trapa bicornis, and revised structure of alnusiin". Heterocycles. 29 (5): 861–864. doi:10.3987/COM-89-4951.
{{cite journal}}: CS1 maint: multiple names: authors list (link) INIST 6780591 - Yoshida T, Yazaki K, Memon M.U, Maruyama I, Kurokawa K, Shingu T and Okuda T (1989). "Structures of alnusiin and bicornin, new hydrolyzable tannins having a monolactonized tergalloyl group". Chemical and Pharmaceutical Bulletin. 37 (10): 2655–2660. doi:10.3987/COM-11-12392.
{{cite journal}}: CS1 maint: multiple names: authors list (link) INIST 19467830 - Yoshida, Takashi; Yazaki, Kazufumi; Memon, Muhammad Usman; Maruyama, Izumi; Kurokawa, Kenji; Shingu, Tetsuro; Okuda, Takuo (1989). "Structures of alnusiin and bicornin, new hydrolyzable tannins having a monolactonized tergalloyl group". Chemical and Pharmaceutical Bulletin. 37 (10): 2655–2660. doi:10.1248/cpb.37.2655.
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |