
Biohydrogen is H2 that is produced biologically. Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass, including biological waste. Furthermore some photosynthetic microorganisms are capable to produce H2 directly from water splitting using light as energy source.
Besides the promising possibilities of biological hydrogen production, many challenges characterize this technology. First challenges include those intrinsic to H2, such as storage and transportation of an explosive noncondensible gas. Additionally, hydrogen producing organisms are poisoned by O2 and yields of H2 are often low.
Biochemical principles
The main reactions driving hydrogen formation involve the oxidation of substrates to obtain electrons. Then, these electrons are transferred to free protons to form molecular hydrogen. This proton reduction reaction is normally performed by an enzyme family known as hydrogenases.
In heterotrophic organisms, electrons are produced during the fermentation of sugars. Hydrogen gas is produced in many types of fermentation as a way to regenerate NAD from NADH. Electrons are transferred to ferredoxin, or can be directly accepted from NADH by a hydrogenase, producing H2. Because of this most of the reactions start with glucose, which is converted to acetic acid.
A related reaction gives formate instead of carbon dioxide:
These reactions are exergonic by 216 and 209 kcal/mol, respectively.
It has been estimated that 99% of all organisms utilize or produce dihydrogen (H2). Most of these species are microbes and their ability to use or produce H2 as a metabolite arises from the expression of H2 metalloenzymes known as hydrogenases. Enzymes within this widely diverse family are commonly sub-classified into three different types based on the active site metal content: -hydrogenases (iron-iron), -hydrogenases (nickel-iron) hydrogenases, and -hydrogenases (iron-only). Many organisms express these enzymes. Notable examples are members of the genera Clostridium, Desulfovibrio, Ralstonia or the pathogen Helicobacter, being most of them strict-anaerobes or facultative microorganisms. Other microorganisms such green algae also express highly active hydrogenases, as it is the case for members of the genera Chlamydomonas.
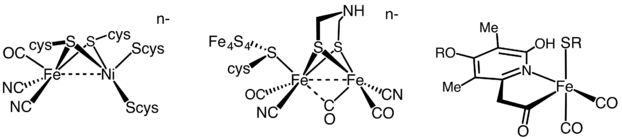
Due to the extreme diversity of hydrogenase enzymes, on-going efforts are focused on screening for novel enzymes with improved features, as well as engineering already characterized hydrogenases to confer them more desirable characteristics.
Production by algae
The biological hydrogen production with algae is a method of photobiological water splitting which is done in a closed photobioreactor based on the production of hydrogen as a solar fuel by algae. Algae produce hydrogen under certain conditions. In 2000 it was discovered that if C. reinhardtii algae are deprived of sulfur they will switch from the production of oxygen, as in normal photosynthesis, to the production of hydrogen.
Green algae express hydrogenases, being some of them considered the most efficient hydrogenases with turnover rates superior to 10 s. This remarkable catalytic efficiency is nonetheless shadowed by its extreme sensitivity to oxygen, being irreversibly inactivated by O2. When the cells are deprived from sulfur, oxygen evolution stops due to photo-damage of photosystem II, in this state the cells start consuming O2 and provide the ideal anaerobic environment for the native hydrogenases to catalyze H2 production.
Photosynthesis
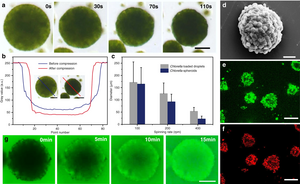
 Algal cell-based bioreactors that can produce hydrogen
Algal cell-based bioreactors that can produce hydrogen
Photosynthesis in cyanobacteria and green algae splits water into hydrogen ions and electrons. The electrons are transported over ferredoxins. Fe-Fe-hydrogenases (enzymes) combine them into hydrogen gas. In Chlamydomonas reinhardtii Photosystem II produces in direct conversion of sunlight 80% of the electrons that end up in the hydrogen gas.
In 2020 scientists reported the development of algal-cell based micro-emulsion for multicellular spheroid microbial reactors capable of producing hydrogen alongside either oxygen or CO2 via photosynthesis in daylight under air. Enclosing the microreactors with synergistic bacteria was shown to increase levels of hydrogen production via reduction of O2 concentrations.
Improving production by light harvesting antenna reduction
The chlorophyll (Chl) antenna size in green algae is minimized, or truncated, to maximize photobiological solar conversion efficiency and H2 production. It has been shown that Light-harvesting complex photosystem II light-harvesting protein LHCBM9 promotes efficient light energy dissipation. The truncated Chl antenna size minimizes absorption and wasteful dissipation of sunlight by individual cells, resulting in better light utilization efficiency and greater photosynthetic efficiency when the green alga are grown as a mass culture in bioreactors.
Economics
With current reports for algae-based biohydrogen, it would take about 25,000 square kilometre algal farming to produce biohydrogen equivalent to the energy provided by gasoline in the US alone. This area represents approximately 10% of the area devoted to growing soya in the US.
Bioreactor design issues
- Restriction of photosynthetic hydrogen production by accumulation of a proton gradient.
- Competitive inhibition of photosynthetic hydrogen production by carbon dioxide.
- Requirement for bicarbonate binding at photosystem II (PSII) for efficient photosynthetic activity.
- Competitive drainage of electrons by oxygen in algal hydrogen production.
- Economics must reach competitive price to other sources of energy and the economics are dependent on several parameters.
- A major technical obstacle is the efficiency in converting solar energy into chemical energy stored in molecular hydrogen.
Attempts are in progress to solve these problems via bioengineering.
Production by cyanobacteria
Biological hydrogen production is also observed in nitrogen-fixing cyanobacteria. This microorganisms can grow forming filaments. Under nitrogen-limited conditions some cells can specialize and form heterocysts, which ensures an anaerobic intracellular space to ease N2 fixation by the nitrogenase enzyme expressed also inside.
Under nitrogen-fixation conditions, the nitrogenase enzyme accepts electrons and consume ATP to break the triple dinitrogen bond and reduce it to ammonia. During the catalytic cycle of the nitrogenase enzyme, molecular hydrogen is also produced.
Nevertheless, since the production of H2 is an important loss of energy for the cells, most of nitrogen fixing cyanobacteria also feature at least one uptake hydrogenase. Uptake hydrogenases exhibit a catalytic bias towards oxygen oxidation, thus can assimilate the produced H2 as a way to recover part of the energy invested during the nitrogen fixation process.
History
In 1933, Marjory Stephenson and her student Stickland reported that cell suspensions catalysed the reduction of methylene blue with H2. Six years later, Hans Gaffron observed that the green photosynthetic alga Chlamydomonas reinhardtii, would sometimes produce hydrogen. In the late 1990s Anastasios Melis discovered that deprivation of sulfur induces the alga to switch from the production of oxygen (normal photosynthesis) to the production of hydrogen. He found that the enzyme responsible for this reaction is hydrogenase, but that the hydrogenase lost this function in the presence of oxygen. Melis also discovered that depleting the amount of sulfur available to the algae interrupted their internal oxygen flow, allowing the hydrogenase an environment in which it can react, causing the algae to produce hydrogen. Chlamydomonas moewusii is also a promising strain for the production of hydrogen.
Industrial hydrogen
Competing for biohydrogen, at least for commercial applications, are many mature industrial processes. Steam reforming of natural gas - sometimes referred to as steam methane reforming (SMR) - is the most common method of producing bulk hydrogen at about 95% of the world production.
See also
- Algaculture – Aquaculture involving the farming of algae
- Hydrogen production – Industrial production of molecular hydrogen
- Hydrogenase – class of enzymes that catalyse the reversible oxidation of molecular hydrogenPages displaying wikidata descriptions as a fallback
- Photohydrogen – Hydrogen produced via light
- Timeline of hydrogen technologies
References
- M. Rögner, ed. (2015). Biohydrogen. De Gruyter. ISBN 978-3-11-033673-3.
- Y.-H. Percival Zhang "Hydrogen Production from Carbohydrates: A Mini-Review" in "Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass" ACS Symposium Series, 2011, Volume 1067, pages=203-216.
- Wijayasekera, Sachindra Chamode; Hewage, Kasun; Siddiqui, Osamah; Hettiaratchi, Patrick; Sadiq, Rehan (2022-01-29). "Waste-to-hydrogen technologies: A critical review of techno-economic and socio-environmental sustainability". International Journal of Hydrogen Energy. 47 (9): 5842–5870. Bibcode:2022IJHE...47.5842W. doi:10.1016/j.ijhydene.2021.11.226. ISSN 0360-3199. S2CID 245348607.
- Bolatkhan, Kenzhegul; Kossalbayev, Bekzhan D.; Zayadan, Bolatkhan K.; Tomo, Tatsuya; Veziroglu, T. Nejat; Allakhverdiev, Suleyman I. (2019-03-01). "Hydrogen production from phototrophic microorganisms: Reality and perspectives". International Journal of Hydrogen Energy. 44 (12): 5799–5811. Bibcode:2019IJHE...44.5799B. doi:10.1016/j.ijhydene.2019.01.092. ISSN 0360-3199. S2CID 104465557.
- Vasiliadou, Ioanna A.; Berná, Antonio; Manchon, Carlos; Melero, Juan A.; Martinez, Fernando; Esteve-Nuñez, Abraham; Puyol, Daniel (2018). "Biological and Bioelectrochemical Systems for Hydrogen Production and Carbon Fixation Using Purple Phototrophic Bacteria". Frontiers in Energy Research. 6. doi:10.3389/fenrg.2018.00107. ISSN 2296-598X.
- Thauer, R. K. (1998). "Biochemistry of Methanogenesis: a Tribute to Marjory Stephenson". Microbiology. 144: 2377–2406. doi:10.1099/00221287-144-9-2377. PMID 9782487.
- Lubitz, Wolfgang; Ogata, Hideaki; Rüdiger, Olaf; Reijerse, Edward (2014). "Hydrogenases". Chemical Reviews. 114 (8): 4081–148. doi:10.1021/cr4005814. PMID 24655035.
- Vignais, Paulette M.; Billoud, Bernard (2007-10-01). "Occurrence, Classification, and Biological Function of Hydrogenases: An Overview". Chemical Reviews. 107 (10): 4206–4272. doi:10.1021/cr050196r. ISSN 0009-2665. PMID 17927159.
- Land, Henrik; Ceccaldi, Pierre; Mészáros, Lívia S.; Lorenzi, Marco; Redman, Holly J.; Senger, Moritz; Stripp, Sven T.; Berggren, Gustav (2019-11-06). "Discovery of novel [FeFe]-hydrogenases for biocatalytic H2-production". Chemical Science. 10 (43): 9941–9948. doi:10.1039/C9SC03717A. ISSN 2041-6539. PMC 6984386. PMID 32055351.
- Grinter, Rhys; Kropp, Ashleigh; Venugopal, Hari; Senger, Moritz; Badley, Jack; Cabotaje, Princess R.; Jia, Ruyu; Duan, Zehui; Huang, Ping; Stripp, Sven T.; Barlow, Christopher K.; Belousoff, Matthew; Shafaat, Hannah S.; Cook, Gregory M.; Schittenhelm, Ralf B. (March 2023). "Structural basis for bacterial energy extraction from atmospheric hydrogen". Nature. 615 (7952): 541–547. Bibcode:2023Natur.615..541G. doi:10.1038/s41586-023-05781-7. ISSN 1476-4687. PMC 10017518. PMID 36890228.
- Morra, Simone (2022). "Fantastic [FeFe]-Hydrogenases and Where to Find Them". Frontiers in Microbiology. 13: 853626. doi:10.3389/fmicb.2022.853626. ISSN 1664-302X. PMC 8924675. PMID 35308355.
- ^ Lu, Yuan; Koo, Jamin (November 2019). "O2 sensitivity and H2 production activity of hydrogenases-A review". Biotechnology and Bioengineering. 116 (11): 3124–3135. doi:10.1002/bit.27136. ISSN 1097-0290. PMID 31403182. S2CID 199539477.
- 2013 - Gimpel JA, et al Advances in microalgae engineering and synthetic biology applications for biofuel production
- Hemschemeier, Anja; Melis, Anastasios; Happe, Thomas (2009). "Analytical approaches to photobiological hydrogen production in unicellular green algae". Photosynthesis Research. 102 (2–3): 523–540. Bibcode:2009PhoRe.102..523H. doi:10.1007/s11120-009-9415-5. ISSN 0166-8595. PMC 2777220. PMID 19291418.
- Wired-Mutant Algae Is Hydrogen Factory Archived August 27, 2006, at the Wayback Machine
- "Further reading - New Scientist". Archived from the original on 2008-10-31. Retrieved 2009-03-11.
- Melis, Anastasios; Zhang, Liping; Forestier, Marc; Ghirardi, Maria L.; Seibert, Michael (2000-01-01). "Sustained Photobiological Hydrogen Gas Production upon Reversible Inactivation of Oxygen Evolution in the Green AlgaChlamydomonas reinhardtii". Plant Physiology. 122 (1): 127–136. doi:10.1104/pp.122.1.127. ISSN 1532-2548. PMC 58851. PMID 10631256.
- ^ Xu, Zhijun; Wang, Shengliang; Zhao, Chunyu; Li, Shangsong; Liu, Xiaoman; Wang, Lei; Li, Mei; Huang, Xin; Mann, Stephen (25 November 2020). "Photosynthetic hydrogen production by droplet-based microbial micro-reactors under aerobic conditions". Nature Communications. 11 (1): 5985. Bibcode:2020NatCo..11.5985X. doi:10.1038/s41467-020-19823-5. ISSN 2041-1723. PMC 7689460. PMID 33239636.
 Available under CC BY 4.0.
Available under CC BY 4.0.
- Peden, E. A.; Boehm, M.; Mulder, D. W.; Davis, R.; Old, W. M.; King, P. W.; Ghirardi, M. L.; Dubini, A. (2013). "Identification of Global Ferredoxin Interaction Networks in Chlamydomonas reinhardtii". Journal of Biological Chemistry. 288 (49): 35192–35209. doi:10.1074/jbc.M113.483727. ISSN 0021-9258. PMC 3853270. PMID 24100040.
- Volgusheva, A.; Styring, S.; Mamedov, F. (2013). "Increased photosystem II stability promotes H2 production in sulfur-deprived Chlamydomonas reinhardtii". Proceedings of the National Academy of Sciences. 110 (18): 7223–7228. Bibcode:2013PNAS..110.7223V. doi:10.1073/pnas.1220645110. ISSN 0027-8424. PMC 3645517. PMID 23589846.
- "Research creates hydrogen-producing living droplets, paving way for alternative future energy source". phys.org. Retrieved 9 December 2020.
- Grewe, S.; Ballottari, M.; Alcocer, M.; D'Andrea, C.; Blifernez-Klassen, O.; Hankamer, B.; Mussgnug, J. H.; Bassi, R.; Kruse, O. (2014). "Light-Harvesting Complex Protein LHCBM9 Is Critical for Photosystem II Activity and Hydrogen Production in Chlamydomonas reinhardtii". The Plant Cell. 26 (4): 1598–1611. Bibcode:2014PlanC..26.1598G. doi:10.1105/tpc.114.124198. ISSN 1040-4651. PMC 4036574. PMID 24706511.
- Kirst, H.; Garcia-Cerdan, J. G.; Zurbriggen, A.; Ruehle, T.; Melis, A. (2012). "Truncated Photosystem Chlorophyll Antenna Size in the Green Microalga Chlamydomonas reinhardtii upon Deletion of the TLA3-CpSRP43 Gene". Plant Physiology. 160 (4): 2251–2260. doi:10.1104/pp.112.206672. ISSN 0032-0889. PMC 3510145. PMID 23043081.
- Growing hydrogen for the cars of tomorrow
- "5.15C: Nitrogen Fixation Mechanism". Biology LibreTexts. 2017-05-11. Retrieved 2023-04-07.
- Tamagnini, Paula; Axelsson, Rikard; Lindberg, Pia; Oxelfelt, Fredrik; Wünschiers, Röbbe; Lindblad, Peter (March 2002). "Hydrogenases and Hydrogen Metabolism of Cyanobacteria". Microbiology and Molecular Biology Reviews. 66 (1): 1–20. doi:10.1128/MMBR.66.1.1-20.2002. ISSN 1092-2172. PMC 120778. PMID 11875125.
- Algae: Power Plant of the Future?
- "Multiplatform Pinup Girl". Wired. 2002-04-01. Archived from the original on 2012-10-20.
- Melis A, Happe T (2001). "Hydrogen Production. Green Algae as a Source of Energy". Plant Physiol. 127 (3): 740–748. doi:10.1104/pp.010498. PMC 1540156. PMID 11706159.
- Yang, Shihui; Guarnieri, Michael T; Smolinski, Sharon; Ghirardi, Maria; Pienkos, Philip T (2013). "De novo transcriptomic analysis of hydrogen production in the green alga Chlamydomonas moewusii through RNA-Seq". Biotechnology for Biofuels. 6 (1): 118. Bibcode:2013BB......6..118Y. doi:10.1186/1754-6834-6-118. ISSN 1754-6834. PMC 3846465. PMID 23971877.
- P. Häussinger, R. Lohmüller, A. M. Watson, "Hydrogen, 2. Production" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.o13_o03
- Ogden, J.M. (1999). "Prospects for building a hydrogen energy infrastructure". Annual Review of Energy and the Environment. 24: 227–279. doi:10.1146/annurev.energy.24.1.227.
- "Hydrogen Production: Natural Gas Reforming". Department of Energy. Retrieved 6 April 2017.
External links
- DOE - A Prospectus for Biological Production of Hydrogen
- FAO
- Maximizing Light Utilization Efficiency and Hydrogen Production in Microalgal Cultures Archived 2013-10-19 at the Wayback Machine
- DIY Algae/Hydrogen Bioreactor 2004
- EERE-CYCLIC PHOTOBIOLOGICAL ALGAL H2-PRODUCTION




