| |

| |

| |
| Names | |
|---|---|
| Other names palladium dichloride bis(acetonitrile), bis(acetonitrile)dichloropalladium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.035.110 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
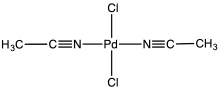
| Chemical formula | C4H6Cl2N2Pd |
| Molar mass | 259.43 g·mol |
| Appearance | yellow-brown |
| Melting point | 129–131 °C (264–268 °F; 402–404 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H301, H311, H330 |
| Precautionary statements | P260, P261, P264, P270, P271, P273, P280, P284, P301+P310, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P310, P311, P312, P320, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Bis(acetonitrile)palladium dichloride is the coordination complex with the formula PdCl2(NCCH3)2. It is the adduct of two acetonitrile ligands with palladium(II) chloride. It is a yellow-brown solid that is soluble in organic solvents. The compound is a reagent and a catalyst for reactions that require soluble Pd(II). The compound is similar to bis(benzonitrile)palladium dichloride. It reacts with 1,5-cyclooctadiene to give dichloro(1,5-cyclooctadiene)palladium.
References
- Carretero, Juan Carlos; Arrayas, Ramon Gomez (2008). "Dichloro bis(acetonitrile) palladium". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00908. ISBN 978-0471936237.
This catalysis article is a stub. You can help Misplaced Pages by expanding it. |