| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name Butanal | |
| Other names Butyraldehyde | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.225 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1129 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H8O |
| Molar mass | 72.107 g·mol |
| Appearance | Colorless liquid |
| Odor | Pungent, aldehyde odor |
| Density | 0.8016 g/mL |
| Melting point | −96.86 °C (−142.35 °F; 176.29 K) |
| Boiling point | 74.8 °C (166.6 °F; 347.9 K) |
| Critical point (T, P) | 537 K (264 °C), 4.32 MPa (42.6 atm) |
| Solubility in water | 7.6 g/100 mL (20 °C) |
| Solubility | Miscible with organic solvents |
| log P | 0.88 |
| Magnetic susceptibility (χ) | −46.08·10 cm/mol |
| Refractive index (nD) | 1.3766 |
| Viscosity | 0.45 cP (20 °C) |
| Dipole moment | 2.72 D |
| Thermochemistry | |
| Heat capacity (C) | 163.7 J·mol·K (liquid) 103.4 J·mol·K (gas) |
| Std molar entropy (S298) |
246.6 J·mol·K (liquid) 343.7 J·mol·K (gas) |
| Std enthalpy of formation (ΔfH298) |
−239.2 kJ·mol (liquid) −204.8 kJ·mol (gas) |
| Std enthalpy of combustion (ΔcH298) |
2470.34 kJ·mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H225, H319 |
| Precautionary statements | P210, P280, P302+P352, P304+P340, P305+P351+P338 |
| NFPA 704 (fire diamond) |
 |
| Flash point | −7 °C (19 °F; 266 K) |
| Autoignition temperature |
230 °C (446 °F; 503 K) |
| Explosive limits | 1.9–12.5% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 2490 mg/kg (rat, oral) |
| Safety data sheet (SDS) | Sigma-Aldrich |
| Related compounds | |
| Related aldehyde | Propionaldehyde Pentanal |
| Related compounds | Butan-1-ol Butyric acid, isobutyraldehyde |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
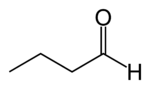
Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colorless flammable liquid with an unpleasant smell. It is miscible with most organic solvents.
Production
Butyraldehyde is produced almost exclusively by the hydroformylation of propylene:
- CH3CH=CH2 + H2 + CO → CH3CH2CH2CHO
Traditionally, hydroformylation was catalyzed by cobalt carbonyl but rhodium complexes are more common. The dominant technology involves the use of rhodium catalysts derived from the water-soluble ligand tppts. An aqueous solution of the rhodium catalyst converts the propylene to the aldehyde, which forms a lighter (less dense) immiscible phase. About 6 billion kilograms are produced annually in this manner. Butyraldehyde can be produced by the catalytic dehydrogenation of n-butanol. At one time, it was produced industrially by the catalytic hydrogenation of crotonaldehyde, which is derived from acetaldehyde.
Reactions and uses
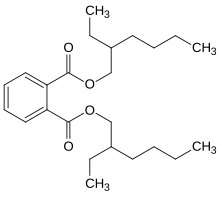
Butyraldehyde undergoes reactions typical of alkyl aldehydes, and these define many of the uses of this compound. Important reactions include hydrogenation to the alcohol, oxidation to the acid, and base-catalyzed condensation. In the presence of a base, two equivalents of butyraldehyde undergoe aldol condensation to give 2-ethylhexenal. This unsaturated aldehyde is then partially hydrogenated to form 2-ethylhexanal, a precursor to plasticizers such as bis(2-ethylhexyl) phthalate.
Butyraldehyde is a component in the two-step synthesis of trimethylolpropane, which is used for the production of alkyd resins.
References
- Merck Index, 11th Edition, 1591.
- CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data. William M. Haynes, David R. Lide, Thomas J. Bruno (2016-2017, 97th ed.). Boca Raton, Florida. 2016. ISBN 978-1-4987-5428-6. OCLC 930681942.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Record of Butyraldehyde in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 13 March 2020.
- ^ Raff, Donald K. (2013). "Butanals". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_447.pub2. ISBN 978-3527306732.
- Werle, Peter; Morawietz, Marcus; Lundmark, Stefan; Sörensen, Kent; Karvinen, Esko; Lehtonen, Juha (2008). "Alcohols, Polyhydric". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_305.pub2. ISBN 978-3-527-30673-2.