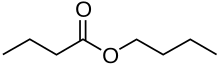
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name Butyl butanoate | |
| Other names
Butyl butyrate 1-Butyl butyrate n-Butyl butyrate n-Butyl n-butyrate Butanoic acid butyl ester Butyric acid butyl ester n-Butyl butanoate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.325 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3082 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H16O2 |
| Molar mass | 144.214 g·mol |
| Density | 0.8692 g/cm at 20 °C |
| Melting point | −91.5 °C (−132.7 °F; 181.7 K) |
| Boiling point | 165 °C (329 °F; 438 K) |
| Solubility in water | insoluble |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H226 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 49 °C (120 °F; 322 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Butyl butyrate, or butyl butanoate, is an organic compound that is an ester formed by the condensation of butyric acid and n-butanol. It is a clear, colorless liquid that is insoluble in water, but miscible with ethanol and diethyl ether. Its refractive index is 1.406 at 20 °C.
Aroma
Like other volatile esters, butyl butyrate has a pleasant aroma. It is used in the flavor industry to create sweet fruity flavors that are similar to that of pineapple. It occurs naturally in many kinds of fruit including apple, banana, berries, pear, plum, and strawberry.
Safety
It is a marine pollutant. It mildly irritates the eyes and skin.
References
- The Merck Index, 12th Edition, 1591
- ^ BUTYL BUTYRATE, at the site cameochemicals.noaa.gov
| Esters | |
|---|---|
| Methyl esters | |
| Ethyl esters | |
| Propyl esters | |
| Butyl esters | |
| Amyl esters | |
| Hexyl esters | |
| Phenyl esters | |
| Heptyl esters | |
| Benzyl esters | |