| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.054 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C22H42O2 |
| Molar mass | 338.576 g·mol |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
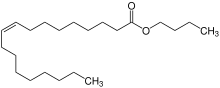
Butyl oleate is a fatty acid ester and an organic chemical found in liquid form. It has the formula C22H42O2 and the CAS Registry Number 142-77-8. It is REACH registered and produced or imported into the European Union with the EC number of 205-559-6.
Synthesis and reactions
It is formed by the condensation of oleic acid and butanol often using an enzyme as catalyst or other biobased catalysts. Ionic liquids may also be used as the catalyst. It undergoes the Bouveault–Blanc reduction with oleyl alcohol and butanol as the products.
Alternative names
It is also known as Butyl cis-9-octadecenoate, Oleic acid butyl ester, butyl 9-octadecenoate and 1-butyl oleate. The IUPAC name is butyl (Z)-octadec-9-enoate.
Uses
It has approval for use as a food additive in Europe and also the US by the FDA. Various other uses include as a lubricant and lubricant additive, paints and coatings additive, and as a plasticizer especially for PVC. Similar to other fatty acid esters, it has found use in biodiesel and as a fuel additive.
See also
References
- "Butyl oleate". pubchem.ncbi.nlm.nih.gov.
- "CAS Common Chemistry". commonchemistry.cas.org. Retrieved 2023-11-10.
- Orrego, Carlos Eduardo; Valencia, Jesús Sigifredo; Zapata, Catalina (2009-05-01). "Candida rugosa Lipase Supported on High Crystallinity Chitosan as Biocatalyst for the Synthesis of 1-Butyl Oleate". Catalysis Letters. 129 (3): 312–322. doi:10.1007/s10562-009-9857-6. ISSN 1572-879X. S2CID 86759909.
- Linko, Y.-Y.; Rantanen, O.; Yu, H. -C.; Linko, P. (1992-01-01). "Factors Affecting Lipase Catalyzed n-Butyl Oleate Synthesis". In Tramper, J.; Vermüe, M. H.; Beeftink, H. H.; von Stockar, U. (eds.). Biocatalysis in Non-Conventional Media. Progress in Biotechnology. Vol. 8. Elsevier. pp. 601–608. doi:10.1016/b978-0-444-89046-7.50087-4. ISBN 9780444890467.
- Leitgeb, M.; Knez, ž. (November 1990). "The influence of water on the synthesis of n‐butyl oleate by immobilized Mucor miehei lipase". Journal of the American Oil Chemists' Society. 67 (11): 775–778. doi:10.1007/BF02540490. ISSN 0003-021X. S2CID 84864739.
- Zhou, Ningning; Yang, Liancheng; Wang, Yuehan; Ding, Yunlong (2022-08-01). "N-butyl oleate catalyzed- synthesized by triethylamine citrate lonic liquid". Journal of Physics: Conference Series. 2321 (1): 012022. Bibcode:2022JPhCS2321a2022Z. doi:10.1088/1742-6596/2321/1/012022. ISSN 1742-6588. S2CID 251491831.
- Wang, Zerong, ed. (2009). "109. Bouveault–Blanc Reduction". Comprehensive Organic Name Reactions and Reagents. pp. 493–496. doi:10.1002/9780470638859.conrr109. ISBN 978-0-471-70450-8.
- PubChem. "Butyl oleate". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-11-10.
- "EU Commission Implementing Regulation adopting the list of flavouring substances". 2012-10-01. Retrieved 2023-11-10.
- "Inventory of Food Contact Substances Listed in 21 CFR". www.cfsanappsexternal.fda.gov. Retrieved 2023-11-10.
- Dailey, Oliver D.; Prevost, Nicolette T.; Strahan, Gary D. (July 2008). "Synthesis and Structural Analysis of Branched‐Chain Derivatives of Methyl Oleate". Journal of the American Oil Chemists' Society. 85 (7): 647–653. doi:10.1007/s11746-008-1235-9. ISSN 0003-021X. S2CID 84876707.
- Riser, G. R.; Bloom, F. W.; Witnauer, L. P. (March 1964). "Evaluation of butyl stearate, butyl oleate, butyl ricinoleate, and methyl oleate as poly(vinyl chloride) plasticizers". Journal of the American Oil Chemists' Society. 41 (3): 172–174. doi:10.1007/BF03024639. ISSN 0003-021X. S2CID 101771799.
- Ghamgui, Hanen; Karra-Chaâbouni, Maha; Gargouri, Youssef (2004-09-01). "1-Butyl oleate synthesis by immobilized lipase from Rhizopus oryzae: a comparative study between n-hexane and solvent-free system". Enzyme and Microbial Technology. 35 (4): 355–363. doi:10.1016/j.enzmictec.2004.06.002. ISSN 0141-0229.
- Lee, Inmok; Johnson, Lawrence A.; Hammond, Earl G. (October 1995). "Use of branched‐chain esters to reduce the crystallization temperature of biodiesel". Journal of the American Oil Chemists' Society. 72 (10): 1155–1160. doi:10.1007/BF02540982. ISSN 0003-021X. S2CID 84340949.