| This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this article by introducing citations to additional sources. Find sources: "Poncirin" – news · newspapers · books · scholar · JSTOR (October 2014) |
| |
| Names | |
|---|---|
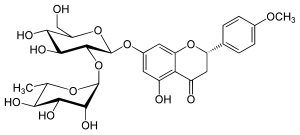
| IUPAC name (2S)-5-Hydroxy-4′-methoxy-7-flavan-4-one | |
| Systematic IUPAC name (2S)-7-{oxy}oxan-2-yl]oxy}-5-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names Isosakuranetin-7-neohesperidoside | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.035.458 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C28H34O14 |
| Molar mass | 594.566 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Poncirin is the 7-O-neohesperidoside of isosakuranetin. Poncirin can be extracted from trifoliate orange (Poncirus trifoliata).
References
- Kim, Dong-Hyun; Bae, Eun-Ah; Han, Myung Joo (1999). "Anti-Helicobacter pylori Activity of the Metabolites of Poncirin from Poncirus trifoliata by Human Intestinal Bacteria". Biological and Pharmaceutical Bulletin. 22 (4): 422–424. doi:10.1248/bpb.22.422. PMID 10328566.
External links
 Media related to Poncirin at Wikimedia Commons
Media related to Poncirin at Wikimedia Commons
| Flavanones and their glycosides | |
|---|---|
| Flavanones | |
| O-methylated flavanones |
|
| C-methylated flavanones | |
| Glycosides |
|
| Acetylated | |
| Acetylated glycosides | |
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |