In biology, chimeric antigen receptors (CARs)—also known as chimeric immunoreceptors, chimeric T cell receptors or artificial T cell receptors—are receptor proteins that have been engineered to give T cells the new ability to target a specific antigen. The receptors are chimeric in that they combine both antigen-binding and T cell activating functions into a single receptor.
CAR T cell therapy uses T cells engineered with CARs to treat cancer. T cells are modified to recognize cancer cells and destroy them. The standard approach is to harvest T cells from patients, genetically alter them, then infuse the resulting CAR T cells into patients to attack their tumors.
CAR T cells can be derived either autologously from T cells in a patient's own blood or allogeneically from those of a donor. Once isolated, these T cells are genetically engineered to express a specific CAR, using a vector derived from an engineered lentivirus such as HIV (see Lentiviral vector in gene therapy). The CAR programs the T cells to target an antigen present on the tumor cell surface. For safety, CAR T cells are engineered to be specific to an antigen that is expressed on a tumor cell but not on healthy cells.
After the modified T cells are infused into a patient, they act as a "living drug" against cancer cells. When they come in contact with their targeted antigen on a cell's surface, T cells bind to it and become activated, then proceed to proliferate and become cytotoxic. CAR T cells destroy cells through several mechanisms, including extensive stimulated cell proliferation, increasing the degree to which they are toxic to other living cells (cytotoxicity), and by causing the increased secretion of factors that can affect other cells such as cytokines, interleukins and growth factors.
The surface of CAR T cells can bear either of two types of co-receptors, CD4 and CD8. These two cell types, called CD4+ and CD8+, respectively, have different and interacting cytotoxic effects. Therapies employing a 1-to-1 ratio of the cell types apparently provide synergistic antitumor effects.

1. T cells are isolated from a patient's blood
2. A new gene encoding a chimeric antigen receptor is incorporated into the T cells
3. Engineered T cells are now specific to a desired target antigen
4. Engineered T cells are expanded in tissue culture
5. Engineered T cells are infused back into the patient
History
The first chimeric receptors containing portions of an antibody and the T cell receptor was described in 1987 by Yoshihisa Kuwana et al. at Fujita Health University and Kyowa Hakko Kogyo, Co. Ltd. in Japan, and independently in 1989 by Gideon Gross and Zelig Eshhar at the Weizmann Institute in Israel. Originally termed "T-bodies", these early approaches combined an antibody's ability to specifically bind to diverse targets with the constant domains of the TCR-α or TCR-β proteins.
In 1991, chimeric receptors containing the intracellular signaling domain of CD3ζ were shown to activate T cell signaling by Arthur Weiss at the University of California, San Francisco. This work prompted CD3ζ intracellular domains to be added to chimeric receptors with antibody-like extracellular domains, commonly single-chain fraction variable (scFv) domains, as well as proteins such as CD4, subsequently termed first generation CARs.
A first generation CAR containing a CD4 extracellular domain and a CD3ζ intracellular domain was used in the first clinical trial of chimeric antigen receptor T cells by the biotechnology company Cell Genesys in the mid 1990s, allowing adoptively transferred T cells to target HIV infected cells, although it failed to show any clinical improvement. Similar early clinical trials of CAR T cells in solid tumors in the 1990s using first generation CARs targeting a solid tumor antigens such as MUC1 did not show long-term persistence of the transferred T cells or result in significant remissions.
In the early 2000s, co-stimulatory domains such as CD28 or 4-1BB were added to first generation CAR's CD3ζ intracellular domain. Termed second generation CARs, these constructs showed greater persistence and improved tumor clearance in pre-clinical models. Clinical trials in the early 2010s using second generation CARs targeting CD19, a protein expressed by normal B cells as well as B-cell leukemias and lymphomas, by investigators at the NCI, University of Pennsylvania, and Memorial Sloan Kettering Cancer Center demonstrated the clinical efficacy of CAR T cell therapies and resulted in complete remissions in many heavily pre-treated patients. These trials ultimately led in the US to the FDA's first two approvals of CAR T cells in 2017, those for tisagenlecleucel (Kymriah), marketed by Novartis originally for B-cell precursor acute lymphoblastic leukemia (B-ALL), and axicabtagene ciloleucel (Yescarta), marketed by Kite Pharma originally for diffuse large B-cell lymphoma (DLBCL). There are now six FDA-approved CAR T therapies.
Production

The first step in the production of CAR T-cells is the isolation of T cells from human blood. CAR T-cells may be manufactured either from the patient's own blood, known as an autologous treatment, or from the blood of a healthy donor, known as an allogeneic treatment. The manufacturing process is the same in both cases; only the choice of initial blood donor is different.
First, leukocytes are isolated using a blood cell separator in a process known as leukocyte apheresis. Peripheral blood mononuclear cells (PBMCs) are then separated and collected. The products of leukocyte apheresis are then transferred to a cell-processing center. In the cell processing center, specific T cells are stimulated so that they will actively proliferate and expand to large numbers. To drive their expansion, T cells are typically treated with the cytokine interleukin 2 (IL-2) and anti-CD3 antibodies. Anti-CD3/CD28 antibodies are also used in some protocols.
The expanded T cells are purified and then transduced with a gene encoding the engineered CAR via a retroviral vector, typically either an integrating gammaretrovirus (RV) or a lentiviral (LV) vector. These vectors are very safe in modern times due to a partial deletion of the U3 region. The new gene editing tool CRISPR/Cas9 has recently been used instead of retroviral vectors to integrate the CAR gene into specific sites in the genome.
The patient undergoes lymphodepletion chemotherapy prior to the introduction of the engineered CAR T-cells. The depletion of the number of circulating leukocytes in the patient upregulates the number of cytokines that are produced and reduces competition for resources, which helps to promote the expansion of the engineered CAR T-cells.
Clinical applications
As of March 2019, there were around 364 ongoing clinical trials happening globally involving CAR T cells. The majority of those trials target blood cancers: CAR T therapies account for more than half of all trials for hematological malignancies. CD19 continues to be the most popular antigen target, followed by BCMA (commonly expressed in multiple myeloma). In 2016, studies began to explore the viability of other antigens, such as CD20. Trials for solid tumors are less dominated by CAR T, with about half of cell therapy-based trials involving other platforms such as NK cells.
Cancer
T cells are genetically engineered to express chimeric antigen receptors specifically directed toward antigens on a patient's tumor cells, then infused into the patient where they attack and kill the cancer cells. Adoptive transfer of T cells expressing CARs is a promising anti-cancer therapeutic, because CAR-modified T cells can be engineered to target potentially any tumor associated antigen.
Early CAR T cell research has focused on blood cancers. The first approved treatments use CARs that target the antigen CD19, present in B-cell-derived cancers such as acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL). There are also efforts underway to engineer CARs targeting many other blood cancer antigens, including CD30 in refractory Hodgkin's lymphoma; CD33, CD123, and FLT3 in acute myeloid leukemia (AML); and BCMA in multiple myeloma.
CAR T cells have also been found to be effective in treating glioblastoma. A single infusion is enough to show rapid tumor regression in a matter of days.
Solid tumors have presented a more difficult target. Identification of good antigens has been challenging: such antigens must be highly expressed on the majority of cancer cells, but largely absent on normal tissues. CAR T cells are also not trafficked efficiently into the center of solid tumor masses, and the hostile tumor microenvironment suppresses T cell activity.
Autoimmune disease
While most CAR T cell studies focus on creating a CAR T cell that can eradicate a certain cell population (for instance, CAR T cells that target lymphoma cells), there are other potential uses for this technology. T cells can also mediate tolerance to antigens. A regulatory T cell outfitted with a CAR could have the potential to confer tolerance to a specific antigen, something that could be utilized in organ transplantation or rheumatologic diseases like lupus.
Approved therapies
| The examples and perspective in this section may not represent a worldwide view of the subject. You may improve this section, discuss the issue on the talk page, or create a new section, as appropriate. (November 2023) (Learn how and when to remove this message) |
| CAR T cell (Brand name) | Company | Approval Agency: Date | Target | Antigen recognition domain | Intracellular signaling domain | Indication (Targeted disease / Line of Therapy) | Agency Product Number, Drug Label |
|---|---|---|---|---|---|---|---|
| tisagenlecleucel
(Kymriah) |
Novartis | FDA: 08/30/2017
EMA: 08/22/2018 MHLW: 05/15/2019 |
CD19 | scFV | 41BB - CD3ζ | B-cell precursor ALL (Third Line)
Diffuse large B-cell lymphoma (Third Line) Follicular lymphoma (Third Line) |
FDA:125646, Label |
| axicabtagene ciloleucel
(Yescarta) |
Kite Pharma / Gilead | FDA: 10/18/2017
EMA: 08/27/2018 NMPA: 06/23/2021 MHLW: 12/22/2022 |
CD19 | scFV | CD28 - CD3ζ | Diffuse large B-cell lymphoma (Second Line)
Follicular lymphoma (Third Line) Primary mediastinal large B-cell lymphoma (Third Line) |
FDA:125643, Label |
| brexucabtagene autoleucel
(Tecartus) |
Kite Pharma / Gilead | FDA: 07/24/2020
EMA: 12/14/2020 |
CD19 | scFV | CD28 - CD3ζ | Mantle cell lymphoma (Third Line)
B-cell precursor ALL (Third Line) |
FDA:125703, Label |
| lisocabtagene maraleucel
(Breyanzi) |
Juno Therapeutics / BMS | FDA: 02/05/2021
EMA: 04/04/2022 MHLW: 12/20/2022 |
CD19 | scFV | 41BB - CD3ζ | Diffuse large B-cell lymphoma (Second Line) | FDA: 25714, Label |
| idecabtagene vicleucel
(Abecma) |
Bluebird Bio / BMS | FDA: 03/26/2021
EMA: 08/18/2021 |
BCMA | scFV | 41BB - CD3ζ | Multiple myeloma (Fourth Line), (Third Line) | FDA:125736, Label |
| ciltacabtagene autoleucel
(Carvykti) |
Janssen / J&J | FDA: 02/28/2022
EMA: 05/25/2022 |
BCMA | VHH | 41BB - CD3ζ | Multiple myeloma (Fourth Line), (Second Line) | FDA:125746, Label |
| obecabtagene autoleucel
(Aucatzyl) |
Autolus | FDA: 11/08/2024 | CD19 | scFV | 41BB - CD3ζ | B-cell precursor ALL (Third Line) | FDA: 125813 Label |
Safety
There are serious side effects that result from CAR T-cells being introduced into the body, including cytokine release syndrome and neurological toxicity. Because it is a relatively new treatment, there are few data about the long-term effects of CAR T-cell therapy. There are still concerns about long-term patient survival, as well as pregnancy complications in female patients treated with CAR T-cells. Anaphylaxis may be a side effect, as the CAR is made with a foreign monoclonal antibody, and as a result provokes an immune response.
On-target/off-tumor recognition occurs when the CAR T-cell recognizes the correct antigen, but the antigen is expressed on healthy, non-pathogenic tissue. This results in the CAR T-cells attacking non-tumor tissue, such as healthy B cells that express CD19 causing B-cell aplasia. The severity of this adverse effect can vary but the combination of prior immunosuppression, lymphodepleting chemotherapy and on-target effects causing hypogammaglobulinaemia and prolonged cytopenias places patients at increased risk of serious infections.
There is also the unlikely possibility that the engineered CAR T-cells will themselves become transformed into cancerous cells through insertional mutagenesis, due to the viral vector inserting the CAR gene into a tumor suppressor or oncogene in the host T cell's genome. Some retroviral (RV) vectors carry a lower risk than lentiviral (LV) vectors. However, both have the potential to be oncogenic. Genomic sequencing analysis of CAR insertion sites in T cells has been established for better understanding of CAR T-cell function and persistence in vivo.
Cytokine release syndrome
Main article: Cytokine release syndromeThe most common issue after treatment with CAR T-cells is cytokine release syndrome (CRS), a condition in which the immune system is activated and releases an increased number of inflammatory cytokines. The clinical manifestation of this syndrome resembles sepsis with high fever, fatigue, myalgia, nausea, capillary leakages, tachycardia and other cardiac dysfunction, liver failure, and kidney impairment. CRS occurs in almost all patients treated with CAR T-cell therapy; in fact, the presence of CRS is a diagnostic marker that indicates the CAR T-cells are working as intended to kill the cancer cells. The severity of CRS does not correlate with an increased response to the treatment, but rather higher disease burden. Severe cytokine release syndrome can be managed with immunosuppressants such as corticosteroids, and with tocilizumab, an anti-IL-6 monoclonal antibody. Early intervention using tocilizumab was shown to reduce the frequency of severe CRS in multiple studies without affecting the therapeutic effect of the treatment. A novel strategy aimed to ameliorate CRS is based on the simultaneous expression of an artificial non-signaling IL-6 receptor on the surface of CAR T-cells. This construct neutralizes macrophage-derived IL-6 through sequestration, thus decreasing the severity of CRS without interfering with the antitumor capability of the CAR T-cell itself.
Immune effector cell-associated neurotoxicity
Neurological toxicity is also often associated with CAR T-cell treatment. The underlying mechanism is poorly understood, and may or may not be related to CRS. Clinical manifestations include delirium, the partial loss of the ability to speak coherently while still having the ability to interpret language (expressive aphasia), lowered alertness (obtundation), and seizures. During some clinical trials, deaths caused by neurotoxicity have occurred. The main cause of death from neurotoxicity is cerebral edema. In a study carried out by Juno Therapeutics, Inc., five patients enrolled in the trial died as a result of cerebral edema. Two of the patients were treated with cyclophosphamide alone and the remaining three were treated with a combination of cyclophosphamide and fludarabine. In another clinical trial sponsored by the Fred Hutchinson Cancer Research Center, there was one reported case of irreversible and fatal neurological toxicity 122 days after the administration of CAR T-cells.
Hypokinetic movement disorder (parkinsonism, or movement and neurocognitive treatment emergent adverse events) has been observed with BCMA-chimeric antigen receptor (CAR) T-cell treatment for multiple myeloma.
Chimeric antigen receptor structure
Chimeric antigen receptors combine many facets of normal T cell activation into a single protein. They link an extracellular antigen recognition domain to an intracellular signalling domain, which activates the T cell when an antigen is bound. CARs are composed of four regions: an antigen recognition domain, an extracellular hinge region, a transmembrane domain, and an intracellular T cell signaling domain.
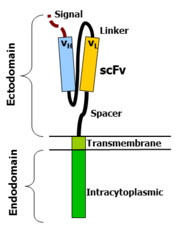
Antigen recognition domain
The antigen recognition domain is exposed to the outside of the cell, in the ectodomain portion of the receptor. It interacts with potential target molecules and is responsible for targeting the CAR T cell to any cell expressing a matching molecule.
The antigen recognition domain is typically derived from the variable regions of a monoclonal antibody linked together as a single-chain variable fragment (scFv). An scFv is a chimeric protein made up of the light (VL) and heavy (VH) chains of immunoglobins, connected with a short linker peptide. These VL and VH regions are selected in advance for their binding ability to the target antigen (such as CD19). The linker between the two chains consists of hydrophilic residues with stretches of glycine and serine in it for flexibility as well as stretches of glutamate and lysine for added solubility. Single domain antibodies (e.g. VH, VHH, VNAR) have been engineered and developed as antigen recognition domains in the CAR format due to their high transduction efficiency in T cells.
In addition to antibody fragments, non-antibody-based approaches have also been used to direct CAR specificity, usually taking advantage of ligand/receptor pairs that normally bind to each other. Cytokines, innate immune receptors, TNF receptors, growth factors, and structural proteins have all been successfully used as CAR antigen recognition domains.
Hinge region
The hinge, also called a spacer, is a small structural domain that sits between the antigen recognition region and the cell's outer membrane. An ideal hinge enhances the flexibility of the scFv receptor head, reducing the spatial constraints between the CAR and its target antigen. This promotes antigen binding and synapse formation between the CAR T cells and target cells. Hinge sequences are often based on membrane-proximal regions from other immune molecules including IgG, CD8, and CD28.
Transmembrane domain
The transmembrane domain is a structural component, consisting of a hydrophobic alpha helix that spans the cell membrane. It anchors the CAR to the plasma membrane, bridging the extracellular hinge and antigen recognition domains with the intracellular signaling region. This domain is essential for the stability of the receptor as a whole. Generally, the transmembrane domain from the most membrane-proximal component of the endodomain is used, but different transmembrane domains result in different receptor stability. The CD28 transmembrane domain is known to result in a highly expressed, stable receptor.
Using the CD3-zeta transmembrane domain is not recommended, as it can result in incorporation of the artificial TCR into the native TCR.
Intracellular T cell signaling domain
The intracellular T cell signaling domain lies in the receptor's endodomain, inside the cell. After an antigen is bound to the external antigen recognition domain, CAR receptors cluster together and transmit an activation signal. Then the internal cytoplasmic end of the receptor perpetuates signaling inside the T cell.
Normal T cell activation relies on the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) present in the cytoplasmic domain of CD3-zeta. To mimic this process, CD3-zeta's cytoplasmic domain is commonly used as the main CAR endodomain component. Other ITAM-containing domains have also been tried, but are not as effective.
T cells also require co-stimulatory molecules in addition to CD3 signaling in order to persist after activation. For this reason, the endodomains of CAR receptors typically also include one or more chimeric domains from co-stimulatory proteins. Signaling domains from a wide variety of co-stimulatory molecules have been successfully tested, including CD28, CD27, CD134 (OX40), and CD137 (4-1BB).
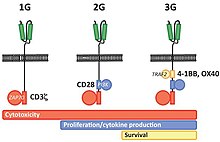
The intracellular signaling domain used defines the generation of a CAR T cell. First generation CARs include only a CD3-zeta cytoplasmic domain. Second generation CARs add a co-stimulatory domain, like CD28 or 4-1BB. The involvement of these intracellular signaling domains improve T cell proliferation, cytokine secretion, resistance to apoptosis, and in vivo persistence. Third generation CARs combine multiple co-stimulatory domains, such as CD28-41BB or CD28-OX40, to augment T cell activity. Preclinical data show the third-generation CARs exhibit improved effector functions and better in vivo persistence as compared to second-generation CARs.
Research directions
Antigen recognition
Although the initial clinical remission rates after CAR T cell therapy in all patients are as high as 90%, long-term survival rates are much lower. The cause is typically the emergence of leukemia cells that do not express CD19 and so evade recognition by the CD19–CAR T cells, a phenomenon known as antigen escape. Preclinical studies developing CAR T cells with dual targeting of CD19 plus CD22 or CD19 plus CD20 have demonstrated promise, and trials studying bispecific targeting to circumvent CD19 down-regulation are ongoing.
In 2018, a version of CAR was developed that is referred to as SUPRA CAR, or split, universal, and programmable. Multiple mechanisms can be deployed to finely regulate the activity of SUPRA CAR, which limits overactivation. In contrast to the traditional CAR design, SUPRA CAR allows targeting of multiple antigens without further genetic modification of a person's immune cells.
Treatment of antigenically heterogeneous tumors can be achieved by administration of a mixture of the desired antigen-specific adaptors.
CAR T function
Fourth generation CARs (also known as TRUCKs or armored CARs) further add factors that enhance T cell expansion, persistence, and anti-tumoral activity. This can include cytokines, such is IL-2, IL-5, IL-12 and co-stimulatory ligands.
Control mechanisms
Adding a synthetic control mechanism to engineered T cells allows doctors to precisely control the persistence or activity of the T cells in the patient's body, with the goal of reducing toxic side effects. The major control techniques trigger T cell death or limit T cell activation, and often regulate the T cells via a separate drug that can be introduced or withheld as needed.
Suicide genes: Genetically modified T cells are engineered to include one or more genes that can induce apoptosis when activated by an extracellular molecule. Herpes simplex virus thymidine kinase (HSV-TK) and inducible caspase 9 (iCasp9) are two types of suicide genes that have been integrated into CAR T cells. In the iCasp9 system, the suicide gene complex has two elements: a mutated FK506-binding protein with high specificity to the small molecule rimiducid/AP1903, and a gene encoding a pro-domain-deleted human caspase 9. Dosing the patient with rimiducid activates the suicide system, leading to rapid apoptosis of the genetically modified T cells. Although both the HSV-TK and iCasp9 systems demonstrate a noticeable function as a safety switch in clinical trials, some defects limit their application. HSV-TK is virus-derived and may be immunogenic to humans. It is also currently unclear whether the suicide gene strategies will act quickly enough in all situations to halt dangerous off-tumor cytotoxicity.
Dual-antigen receptor: CAR T cells are engineered to express two tumor-associated antigen receptors at the same time, reducing the likelihood that the T cells will attack non-tumor cells. Dual-antigen receptor CAR T cells have been reported to have less intense side effects. An in vivo study in mice shows that dual-receptor CAR T cells effectively eradicated prostate cancer and achieved complete long-term survival.
ON-switch and OFF-switch: In this system, CAR T cells can only function in the presence of both tumor antigen and a benign exogenous molecule. To achieve this, the CAR T cell's engineered chimeric antigen receptor is split into two separate proteins that must come together in order to function. The first receptor protein typically contains the extracellular antigen binding domain, while the second protein contains the downstream signaling elements and co-stimulatory molecules (such as CD3ζ and 4-1BB). In the presence of an exogenous molecule (such as a rapamycin analog), the binding and signaling proteins dimerize together, allowing the CAR T cells to attack the tumor. Human EGFR truncated form (hEGFRt) has been used as an OFF-switch for CAR T cells using cetuximab.
Bispecific molecules as switches: Bispecific molecules target both a tumor-associated antigen and the CD3 molecule on the surface of T cells. This ensures that the T cells cannot become activated unless they are in close physical proximity to a tumor cell. The anti-CD20/CD3 bispecific molecule shows high specificity to both malignant B cells and cancer cells in mice. FITC is another bifunctional molecule used in this strategy. FITC can redirect and regulate the activity of the FITC-specific CAR T cells toward tumor cells with folate receptors.
Advances in CAR T cell manufacturing.
Due to the high costs of CAR T cell therapy, a number of alternative efforts are being investigated to improve CAR T cell manufacturing and reduce costs. In vivo CAR T cell manufacturing strategies are being tested. In addition, bioinstructive materials have been developed for CAR T cell generation. Rapid CAR T cell generation is also possible through shortening or eliminating the activation and expansion steps.
In situ modification
Another approach is to modify T cells and/or B cells still in the body using viral vectors.
Alternative Activating Domains
Recent advancements in CAR T-cell therapy have focused on alternative activating domains to enhance efficacy and overcome resistance in solid tumors. For instance, Toll-like receptor 4 (TLR4) signaling components can be incorporated into CAR constructs to modulate cytokine production and boost T-cell activation and proliferation, leading to enhanced CAR T-cell expansion and persistence. Similarly, the FYN kinase, a member of the Src family kinases involved in T-cell receptor signaling, can be integrated to improve the signaling cascade within CAR T-cells, resulting in better targeting and elimination of cancer cells. Additionally, KIR-based CARs (KIR-CAR), which use the transmembrane and intracellular domains of the activating receptor KIR2DS2 combined with the DAP-12 signaling adaptor, have shown improved T-cell proliferation and antitumor activity. These strategies, including the use of nonconventional costimulatory molecules like MyD88/CD40, highlight the innovative approaches being taken to optimize CAR T-cell therapies for more effective cancer treatments.
Economics
The cost of CAR T cell therapies has been criticized, with the initial costs of tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) being $375,000 and $475,000 respectively. The high cost of CAR T therapies is due to complex cellular manufacturing in specialized good manufacturing practice (GMP) facilities as well as the high level of hospital care necessary after CAR T cells are administered due to risks such as cytokine release syndrome. In the United States, CAR T cell therapies are covered by Medicare and by many but not all private insurers. Manufacturers of CAR T cells have developed alternative payment programs due to the high cost of CAR T therapy, such as by requiring payment only if the CAR T therapy induces a complete remission by a certain time point after treatment.
Additionally, CAR T cell therapies are not available worldwide yet. CAR T cell therapies have been approved in China, Australia, Singapore, the United Kingdom, and some European countries. In February 2022 Brazil approved tisagenlecleucel (Kymriah) treatment.
See also
References
- Fox M (July 12, 2017). "New Gene Therapy for Cancer Offers Hope to Those With No Options Left". NBC News.
- Srivastava S, Riddell SR (August 2015). "Engineering CAR-T cells: Design concepts". Trends in Immunology. 36 (8): 494–502. doi:10.1016/j.it.2015.06.004. PMC 4746114. PMID 26169254.
- ^ Sadelain M, Brentjens R, Rivière I (April 2013). "The basic principles of chimeric antigen receptor design". Cancer Discovery. 3 (4): 388–398. doi:10.1158/2159-8290.CD-12-0548. PMC 3667586. PMID 23550147.
- ^ Hartmann J, Schüßler-Lenz M, Bondanza A, Buchholz CJ (September 2017). "Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts". EMBO Molecular Medicine. 9 (9): 1183–1197. doi:10.15252/emmm.201607485. PMC 5582407. PMID 28765140.
- Tang XJ, Sun XY, Huang KM, Zhang L, Yang ZS, Zou DD, et al. (December 2015). "Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy". Oncotarget. 6 (42): 44179–44190. doi:10.18632/oncotarget.6175. PMC 4792550. PMID 26496034.
- Zhang H, Zhao P, Huang H (December 2020). "Engineering better chimeric antigen receptor T cells". Experimental Hematology & Oncology. 9 (1): 34. doi:10.1186/s40164-020-00190-2. PMC 7709221. PMID 33292660.
- Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, et al. (December 1987). "Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions". Biochemical and Biophysical Research Communications. 149 (3): 960–968. doi:10.1016/0006-291x(87)90502-x. PMID 3122749.
- Gross G, Gorochov G, Waks T, Eshhar Z (February 1989). "Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity". Transplantation Proceedings. 21 (1 Pt 1): 127–130. PMID 2784887.
- Rosenbaum L (October 2017). "Tragedy, Perseverance, and Chance - The Story of CAR-T Therapy". The New England Journal of Medicine. 377 (14): 1313–1315. doi:10.1056/NEJMp1711886. PMID 28902570. S2CID 205114161.
- Gross G, Waks T, Eshhar Z (December 1989). "Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity". Proceedings of the National Academy of Sciences of the United States of America. 86 (24): 10024–10028. Bibcode:1989PNAS...8610024G. doi:10.1073/pnas.86.24.10024. JSTOR 34790. PMC 298636. PMID 2513569.
- Eshhar Z, Bach N, Fitzer-Attas CJ, Gross G, Lustgarten J, Waks T, Schindler DG (1996). "The T-body approach: potential for cancer immunotherapy". Springer Seminars in Immunopathology. 18 (2): 199–209. doi:10.1007/BF00820666. PMID 8908700. S2CID 19872173.
- Irving BA, Weiss A (March 1991). "The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways". Cell. 64 (5): 891–901. doi:10.1016/0092-8674(91)90314-o. PMID 1705867. S2CID 23466990.
- ^ Hege KM, Roberts MR (December 1996). "T-cell gene therapy". Current Opinion in Biotechnology. 7 (6): 629–634. doi:10.1016/s0958-1669(96)80074-7. PMID 8939644.
- June CH, Sadelain M (July 2018). "Chimeric Antigen Receptor Therapy". The New England Journal of Medicine. 379 (1): 64–73. doi:10.1056/NEJMra1706169. PMC 7433347. PMID 29972754.
- ^ Braendstrup P, Levine BL, Ruella M (February 2020). "The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19". Cytotherapy. 22 (2): 57–69. doi:10.1016/j.jcyt.2019.12.004. PMC 7036015. PMID 32014447.
- Sadelain M, Rivière I, Brentjens R (January 2003). "Targeting tumours with genetically enhanced T lymphocytes". Nature Reviews. Cancer. 3 (1): 35–45. doi:10.1038/nrc971. PMID 12509765. S2CID 33707802.
- Center for Biologics Evaluation and Research (2022-03-01). "Approved Cellular and Gene Therapy Products". FDA.
- Jin C, Yu D, Hillerdal V, Wallgren A, Karlsson-Parra A, Essand M (2014-03-05). "Allogeneic lymphocyte-licensed DCs expand T cells with improved antitumor activity and resistance to oxidative stress and immunosuppressive factors". Molecular Therapy: Methods & Clinical Development. 1: 14001. doi:10.1038/mtm.2014.1. PMC 4362340. PMID 26015949.
- ^ Li, Nan; Ho, Mitchell (2022). "Development of Glypican-2 Targeting Single-Domain Antibody CAR T Cells for Neuroblastoma". Single-Domain Antibodies. Methods in Molecular Biology. Vol. 2446. pp. 451–468. doi:10.1007/978-1-0716-2075-5_23. ISBN 978-1-0716-2074-8. ISSN 1940-6029. PMID 35157288. S2CID 246813053.
- ^ Makita S, Yoshimura K, Tobinai K (June 2017). "Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma". Cancer Science. 108 (6): 1109–1118. doi:10.1111/cas.13239. PMC 5480083. PMID 28301076.
- Jin C, Fotaki G, Ramachandran M, Nilsson B, Essand M, Yu D (July 2016). "Safe engineering of CAR T cells for adoptive cell therapy of cancer using long-term episomal gene transfer". EMBO Molecular Medicine. 8 (7): 702–711. doi:10.15252/emmm.201505869. PMC 4931286. PMID 27189167.
- Jensen TI, Axelgaard E, Bak RO (June 2019). "Therapeutic gene editing in haematological disorders with CRISPR/Cas9". British Journal of Haematology. 185 (5): 821–835. doi:10.1111/bjh.15851. PMID 30864164.
- Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP (December 2006). "Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go?". Nature Clinical Practice. Oncology. 3 (12): 668–681. doi:10.1038/ncponc0666. PMC 1773008. PMID 17139318.
- ^ Xin Yu J, Hubbard-Lucey VM, Tang J (October 2019). "The global pipeline of cell therapies for cancer". Nature Reviews. Drug Discovery. 18 (11): 821–822. doi:10.1038/d41573-019-00090-z. PMID 31673124. S2CID 190862546.
- Brudno and Kochenderfer. Chimeric antigen receptor T cell therapies for lymphoma. Nature Reviews Clinical Oncology. 2018. 15: 31-46.
- Mikkilineni and Kochenderfer. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017. 130: 2594-602
- Almåsbak H, Aarvak T, Vemuri MC (2016). "CAR T Cell Therapy: A Game Changer in Cancer Treatment". Journal of Immunology Research. 2016: 5474602. doi:10.1155/2016/5474602. PMC 4889848. PMID 27298832.
- Jacobson CA, Ritz J (November 2011). "Time to put the CAR-T before the horse". Blood. 118 (18): 4761–4762. doi:10.1182/blood-2011-09-376137. PMID 22053170.
- Li, Nan; Spetz, Madeline R.; Li, Dan; Ho, Mitchell (July 2021). "Advances in immunotherapeutic targets for childhood cancers: A focus on glypican-2 and B7-H3". Pharmacology & Therapeutics. 223: 107892. doi:10.1016/j.pharmthera.2021.107892. ISSN 1879-016X. PMC 8202769. PMID 33992682.
- ^ Li, Dan; Lin, Shaoli; Hong, Jessica; Ho, Mitchell (2022). "Immunotherapy for hepatobiliary cancers: Emerging targets and translational advances". Advances in Cancer Research. 156: 415–449. doi:10.1016/bs.acr.2022.01.013. ISBN 9780323983921. ISSN 2162-5557. PMID 35961708. S2CID 246978004.
- Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. (November 2010). "Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19". Blood. 116 (20): 4099–4102. doi:10.1182/blood-2010-04-281931. PMC 2993617. PMID 20668228.
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. (February 2015). "Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor". Journal of Clinical Oncology. 33 (6): 540–549. doi:10.1200/JCO.2014.56.2025. PMC 4322257. PMID 25154820.
- ^ Schultz L, Mackall C (February 2019). "Driving CAR T cell translation forward". Science Translational Medicine. 11 (481): eaaw2127. doi:10.1126/scitranslmed.aaw2127. PMID 30814337.
- Choi, Bryan D.; Gerstner, Elizabeth R.; Frigault, Matthew J.; Leick, Mark B.; Mount, Christopher W.; Balaj, Leonora; Nikiforow, Sarah; Carter, Bob S.; Curry, William T.; Gallagher, Kathleen; Maus, Marcela V. (2024-04-11). "Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma". New England Journal of Medicine. 390 (14): 1290–1298. doi:10.1056/NEJMoa2314390. ISSN 0028-4793. PMC 11162836. PMID 38477966.
- Lim WA, June CH (February 2017). "The Principles of Engineering Immune Cells to Treat Cancer". Cell. 168 (4): 724–740. doi:10.1016/j.cell.2017.01.016. PMC 5553442. PMID 28187291.
- ^ Li D, Li N, Zhang YF, Fu H, Feng M, Schneider D, et al. (June 2020). "Persistent Polyfunctional Chimeric Antigen Receptor T Cells That Target Glypican 3 Eliminate Orthotopic Hepatocellular Carcinomas in Mice". Gastroenterology. 158 (8): 2250–2265.e20. doi:10.1053/j.gastro.2020.02.011. PMC 7282931. PMID 32060001.
- Li, Nan; Fu, Haiying; Hewitt, Stephen M.; Dimitrov, Dimiter S.; Ho, Mitchell (2017-08-08). "Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma". Proceedings of the National Academy of Sciences of the United States of America. 114 (32): E6623–E6631. Bibcode:2017PNAS..114E6623L. doi:10.1073/pnas.1706055114. ISSN 1091-6490. PMC 5559039. PMID 28739923.
- ^ Li, Nan; Torres, Madeline B.; Spetz, Madeline R.; Wang, Ruixue; Peng, Luyi; Tian, Meijie; Dower, Christopher M.; Nguyen, Rosa; Sun, Ming; Tai, Chin-Hsien; de Val, Natalia; Cachau, Raul; Wu, Xiaolin; Hewitt, Stephen M.; Kaplan, Rosandra N. (2021-06-15). "CAR T cells targeting tumor-associated exons of glypican 2 regress neuroblastoma in mice". Cell Reports. Medicine. 2 (6): 100297. doi:10.1016/j.xcrm.2021.100297. ISSN 2666-3791. PMC 8233664. PMID 34195677.
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M (May 2008). "Regulatory T cells and immune tolerance". Cell. 133 (5): 775–787. doi:10.1016/j.cell.2008.05.009. PMID 18510923. S2CID 2315895.
- Zhang Q, Lu W, Liang CL, Chen Y, Liu H, Qiu F, Dai Z (2018). "Chimeric Antigen Receptor (CAR) Treg: A Promising Approach to Inducing Immunological Tolerance". Frontiers in Immunology. 9: 2359. doi:10.3389/fimmu.2018.02359. PMC 6194362. PMID 30369931.
- Mougiakakos D, Krönke G, Völkl S, Kretschmann S, Aigner M, Kharboutli S, et al. (August 2021). "CD19-Targeted CAR T Cells in Refractory Systemic Lupus Erythematosus". The New England Journal of Medicine. 385 (6): 567–569. doi:10.1056/NEJMc2107725. PMID 34347960. S2CID 236927691.
- ^ "Novartis receives first ever FDA approval for a CAR-T cell therapy, Kymriah(TM) (CTL019), for children and young adults with B-cell ALL that is refractory or has relapsed at least twice". www.novartis.com. 2017-08-30.
- ^ "Novartis receives European Commission approval of its CAR-T cell therapy, Kymriah® (tisagenlecleucel)". www.novartis.com. Retrieved 2023-11-18.
- ^ "Novartis gets approval to sell Kymriah in Japan for $306,000". www.reuters.com. 2019-05-15.
- "KYMRIAH (tisagenlecleucel)". US Food and Drug Administration. 2019-04-05.
- "FDA approves Novartis Kymriah® CAR-T cell therapy for adult patients with relapsed or refractory follicular lymphoma". Novartis. Retrieved 2022-06-05.
- "Novartis Kymriah® receives EC approval as first CAR-T cell therapy for adults with relapsed or refractory follicular lymphoma". www.novartis.com. Retrieved 2023-11-18.
- Center for Biologics Evaluation and Research (2017-10-18). "FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma". FDA.
- ^ "Yescarta® (Axicabtagene Ciloleucel) Receives European Marketing Authorization for the Treatment of Relapsed or Refractory DLBCL and PMBCL, After Two or More Lines of Systemic Therapy". www.gilead.com. Retrieved 2023-11-18.
- ^ "Kite Joint Venture - Fosun Kite - Gains the First CAR T-cell Therapy Approval in China". www.gilead.com. Retrieved 2023-11-13.
- ^ "Yescarta® Now Approved in Japan for Initial Treatment of Relapsed/Refractory Large B-Cell Lymphoma". www.gilead.com. 2022-12-22.
- Center for Biologics Evaluation and Research (2020-05-28). "YESCARTA (axicabtagene ciloleucel)". FDA.
- "Kite's CAR T-cell Therapy Yescarta® First in Europe to Receive Positive CHMP Opinion for Use in Second-line Diffuse Large B-cell Lymphoma and High-grade B-cell Lymphoma". www.gilead.com. Retrieved 2023-11-18.
- "U.S. FDA Approves Yescarta® for Relapsed or Refractory Follicular Lymphoma After Two or More Lines of Systemic Therapy". www.gilead.com. Retrieved 2021-03-15.
- "Kite's CAR T-cell Therapy Yescarta® Granted European Marketing Authorization for the Treatment of Relapsed or Refractory Follicular Lymphoma". www.gilead.com. Retrieved 2023-11-18.
- Center for Biologics Evaluation and Research (2021-03-04). "TECARTUS (brexucabtagene autoleucel)". FDA.
- ^ European Medicines Agency (2023-01-30). "TECARTUS (brexucabtagene autoleucel)". EMA.
- ^ Center for Biologics Evaluation and Research (2021-03-04). "TECARTUS (brexucabtagene autoleucel)". FDA.
- "U.S. Food and Drug Administration Approves Bristol Myers Squibb's Breyanzi (lisocabtagene maraleucel), a New CAR T Cell Therapy for Adults with Relapsed or Refractory Large B-cell Lymphoma". news.bms.com. Retrieved 2023-11-21.
- ^ European Medicines Agency (2023-11-10). "Breyanzi". EMA.
- ^ "CAR T Cell Therapy Breyanzi® Approved as Relapsed or Refractory Large B-cell Lymphoma Second-Line Therapy in Japan". new.bms.com.
- Center for Biologics Evaluation and Research (2022-06-24). "FDA D.I.S.C.O. Burst Edition: FDA approval of Breyanzi (lisocabtagene maraleucel) for second-line treatment of large B-cell lymphoma". FDA.
- ^ Center for Biologics Evaluation and Research (2021-03-27). "ABECMA (idecabtagene vicleucel)". FDA.
- ^ European Medicines Agency (2023-07-27). "Abecma". EMA.
- ^ Center for Biologics Evaluation and Research (2022-03-21). "CARVYKTI". FDA.
- ^ European Medicines Agency (2023-07-27). "Carvykti". EMA.
- Research, Center for Drug Evaluation and (2024-11-08). "FDA approves obecabtagene autoleucel for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia". FDA.
- ^ Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ (2016). "Toxicity and management in CAR T-cell therapy". Molecular Therapy: Oncolytics. 3: 16011. doi:10.1038/mto.2016.11. PMC 5008265. PMID 27626062.
- Bupha-Intr O, Haeusler G, Chee L, Thursky K, Slavin M, Teh B (June 2021). "CAR-T cell therapy and infection: a review". Expert Review of Anti-Infective Therapy. 19 (6): 749–758. doi:10.1080/14787210.2021.1855143. PMID 33249873. S2CID 227235627.
- Breslin S (February 2007). "Cytokine-release syndrome: overview and nursing implications". Clinical Journal of Oncology Nursing. 11 (1 Suppl): 37–42. doi:10.1188/07.CJON.S1.37-42. PMID 17471824. S2CID 35773028.
- Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. (July 2014). "Current concepts in the diagnosis and management of cytokine release syndrome". Blood. 124 (2): 188–195. doi:10.1182/blood-2014-05-552729. PMC 4093680. PMID 24876563.
- Berg P, Schönefeld S, Ruppert-Seipp G, Funk MB (2022-11-29). "Regulatory Measures to Improve the Safety of CAR-T-Cell Treatment". Transfusion Medicine and Hemotherapy. 50 (3): 218–225. doi:10.1159/000526786. ISSN 1660-3796. PMC 10331154. PMID 37435000.
- Gardner RA, Ceppi F, Rivers J, Annesley C, Summers C, Taraseviciute A, et al. (December 2019). "Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy". Blood. 134 (24): 2149–2158. doi:10.1182/blood.2019001463. PMC 6908832. PMID 31697826.
- Tan AH, Vinanica N, Campana D (April 2020). "Chimeric antigen receptor-T cells with cytokine neutralizing capacity". Blood advances. 4 (7): 1419–1431. doi:10.1182/bloodadvances.2019001287. PMC 7160280. PMID 32271901.
- Brudno JN, Kochenderfer JN (June 2016). "Toxicities of chimeric antigen receptor T cells: recognition and management". Blood. 127 (26): 3321–3330. doi:10.1182/blood-2016-04-703751. PMC 4929924. PMID 27207799.
- "Study Evaluating the Efficacy and Safety of JCAR015 in Adult B-cell Acute Lymphoblastic Leukemia (B-ALL)". ClinicalTrials.gov. Retrieved 2018-02-21.
- Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. (June 2016). "CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients". The Journal of Clinical Investigation. 126 (6): 2123–2138. doi:10.1172/JCI85309. PMC 4887159. PMID 27111235.
- Gust, Juliane (October 5, 2023). "BCMA-CAR T-cell treatment–associated parkinsonism". Blood. 142 (14): 1181–1183. doi:10.1182/blood.2023021860. PMID 37796518. Retrieved April 4, 2024.
- ^ Chandran SS, Klebanoff CA (July 2019). "T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance". Immunological Reviews. 290 (1): 127–147. doi:10.1111/imr.12772. PMC 7027847. PMID 31355495.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Dotti G, Gottschalk S, Savoldo B, Brenner MK (January 2014). "Design and development of therapies using chimeric antigen receptor-expressing T cells". Immunological Reviews. 257 (1): 107–126. doi:10.1111/imr.12131. PMC 3874724. PMID 24329793.
- ^ Zhang C, Liu J, Zhong JF, Zhang X (2017-06-24). "Engineering CAR-T cells". Biomarker Research. 5: 22. doi:10.1186/s40364-017-0102-y. PMC 5482931. PMID 28652918.
- Baldo BA (May 2015). "Chimeric fusion proteins used for therapy: indications, mechanisms, and safety". Drug Safety. 38 (5): 455–479. doi:10.1007/s40264-015-0285-9. PMID 25832756. S2CID 23852865.
- Li N, Fu H, Hewitt SM, Dimitrov DS, Ho M (August 2017). "Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma". Proceedings of the National Academy of Sciences of the United States of America. 114 (32): E6623–E6631. Bibcode:2017PNAS..114E6623L. doi:10.1073/pnas.1706055114. PMC 5559039. PMID 28739923.
- ^ Li, Nan; Quan, Alex; Li, Dan; Pan, Jiajia; Ren, Hua; Hoeltzel, Gerard; de Val, Natalia; Ashworth, Dana; Ni, Weiming; Zhou, Jing; Mackay, Sean; Hewitt, Stephen M.; Cachau, Raul; Ho, Mitchell (2023-04-08). "The IgG4 hinge with CD28 transmembrane domain improves VHH-based CAR T cells targeting a membrane-distal epitope of GPC1 in pancreatic cancer". Nature Communications. 14 (1): 1986. Bibcode:2023NatCo..14.1986L. doi:10.1038/s41467-023-37616-4. ISSN 2041-1723. PMC 10082787. PMID 37031249.
- ^ Kolluri, Aarti; Li, Dan; Li, Nan; Duan, Zhijian; Roberts, Lewis R.; Ho, Mitchell (2023-02-01). "Human VH-based chimeric antigen receptor T cells targeting glypican 3 eliminate tumors in preclinical models of HCC". Hepatology Communications. 7 (2): e0022. doi:10.1097/HC9.0000000000000022. ISSN 2471-254X. PMC 9851680. PMID 36691969.
- Li, Dan; English, Hejiao; Hong, Jessica; Liang, Tianyuzhou; Merlino, Glenn; Day, Chi-Ping; Ho, Mitchell (2022-03-17). "A novel PD-L1-targeted shark VNAR single-domain-based CAR-T cell strategy for treating breast cancer and liver cancer". Molecular Therapy: Oncolytics. 24: 849–863. doi:10.1016/j.omto.2022.02.015. ISSN 2372-7705. PMC 8917269. PMID 35317524.
- Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, et al. (February 2015). "The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity". Cancer Immunology Research. 3 (2): 125–135. doi:10.1158/2326-6066.CIR-14-0127. PMC 4692801. PMID 25212991.
- Qin L, Lai Y, Zhao R, Wei X, Weng J, Lai P, et al. (March 2017). "Incorporation of a hinge domain improves the expansion of chimeric antigen receptor T cells". Journal of Hematology & Oncology. 10 (1): 68. doi:10.1186/s13045-017-0437-8. PMC 5347831. PMID 28288656.
- Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE (June 2010). "The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex". Journal of Immunology. 184 (12): 6938–6949. doi:10.4049/jimmunol.0901766. PMID 20483753.
- Casucci M, Bondanza A (2011). "Suicide gene therapy to increase the safety of chimeric antigen receptor-redirected T lymphocytes". Journal of Cancer. 2: 378–382. doi:10.7150/jca.2.378. PMC 3133962. PMID 21750689.
- "A Cure for Cancer? How CAR-T Therapy is Revolutionizing Oncology" (Press release). labiotech. March 8, 2018. Retrieved April 19, 2018.
- Choe JH, Williams JZ, Lim WA (2020). "Engineering T Cells to Treat Cancer: The Convergence of Immuno-Oncology and Synthetic Biology". Annual Review of Cancer Biology. 4: 121–139. doi:10.1146/annurev-cancerbio-030419-033657.
- Cho JH, Collins JJ, Wong WW (May 2018). "Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses". Cell. 173 (6): 1426–1438.e11. doi:10.1016/j.cell.2018.03.038. PMC 5984158. PMID 29706540.
- SMDC technology. Archived 2016-03-27 at the Wayback Machine ENDOCYTE
- "Endocyte announces promising preclinical data for application of SMDC technology in CAR T cell therapy in late-breaking abstract at American Association for Cancer Research (AACR) annual meeting 2016" (Press release). Endocyte. April 19, 2016. Archived from the original on July 30, 2017. Retrieved December 20, 2017.
- Kueberuwa G, Kalaitsidou M, Cheadle E, Hawkins RE, Gilham DE (March 2018). "CD19 CAR T Cells Expressing IL-12 Eradicate Lymphoma in Fully Lymphoreplete Mice through Induction of Host Immunity". Molecular Therapy: Oncolytics. 8: 41–51. doi:10.1016/j.omto.2017.12.003. PMC 5772011. PMID 29367945.
- Chmielewski M, Abken H (2015). "TRUCKs: the fourth generation of CARs". Expert Opinion on Biological Therapy. 15 (8): 1145–1154. doi:10.1517/14712598.2015.1046430. PMID 25985798. S2CID 42535203.
- ^ Zhang E, Xu H (January 2017). "A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy". Journal of Hematology & Oncology. 10 (1): 1. doi:10.1186/s13045-016-0379-6. PMC 5210295. PMID 28049484.
- Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, et al. (June 1997). "HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia". Science. 276 (5319): 1719–1724. doi:10.1126/science.276.5319.1719. PMID 9180086.
- Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. (October 2007). "Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes". Blood. 110 (8): 2793–2802. doi:10.1182/blood-2007-02-072843. PMC 2018664. PMID 17638856.
- Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA, et al. (February 1996). "T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients". Nature Medicine. 2 (2): 216–223. doi:10.1038/nm0296-216. PMID 8574968. S2CID 35503876.
- Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M (January 2002). "Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor". Nature Biotechnology. 20 (1): 70–75. doi:10.1038/nbt0102-70. PMID 11753365. S2CID 20302096.
- Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, et al. (October 2012). "Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling". Journal of Clinical Immunology. 32 (5): 1059–1070. doi:10.1007/s10875-012-9689-9. PMID 22526592. S2CID 17660404.
- Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA (October 2015). "Remote control of therapeutic T cells through a small molecule-gated chimeric receptor". Science. 350 (6258): aab4077. Bibcode:2015Sci...350.4077W. doi:10.1126/science.aab4077. PMC 4721629. PMID 26405231.
- Frankel SR, Baeuerle PA (June 2013). "Targeting T cells to tumor cells using bispecific antibodies". Current Opinion in Chemical Biology. 17 (3): 385–392. doi:10.1016/j.cbpa.2013.03.029. PMID 23623807.
- Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, et al. (May 2015). "Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies". Science Translational Medicine. 7 (287): 287ra70. doi:10.1126/scitranslmed.aaa4802. PMID 25972002. S2CID 24939667.
- Kim CH, Axup JY, Lawson BR, Yun H, Tardif V, Choi SH, et al. (October 2013). "Bispecific small molecule-antibody conjugate targeting prostate cancer". Proceedings of the National Academy of Sciences of the United States of America. 110 (44): 17796–17801. Bibcode:2013PNAS..11017796K. doi:10.1073/pnas.1316026110. PMC 3816437. PMID 24127589.
- ^ Lyman GH, Nguyen A, Snyder S, Gitlin M, Chung KC (April 2020). "Economic Evaluation of Chimeric Antigen Receptor T-Cell Therapy by Site of Care Among Patients With Relapsed or Refractory Large B-Cell Lymphoma". JAMA Network Open. 3 (4): e202072. doi:10.1001/jamanetworkopen.2020.2072. PMC 7136832. PMID 32250433.
- Smith, Tyrel T.; Stephan, Sirkka B.; Moffett, Howell F.; McKnight, Laura E.; Ji, Weihang; Reiman, Diana; Bonagofski, Emmy; Wohlfahrt, Martin E.; Pillai, Smitha P. S.; Stephan, Matthias T. (2017-04-17). "In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers". Nature Nanotechnology. 12 (8): 813–820. doi:10.1038/nnano.2017.57. ISSN 1748-3387. PMC 5646367. PMID 28416815.
- Agarwal, Shiwani; Weidner, Tatjana; Thalheimer, Frederic B.; Buchholz, Christian J. (2019-10-10). "In vivo generated human CAR T cells eradicate tumor cells". OncoImmunology. 8 (12): e1671761. doi:10.1080/2162402x.2019.1671761. ISSN 2162-402X. PMC 6844313. PMID 31741773.
- Agarwalla, Pritha; Ogunnaike, Edikan A.; Ahn, Sarah; Froehlich, Kristen A.; Jansson, Anton; Ligler, Frances S.; Dotti, Gianpietro; Brudno, Yevgeny (2022-03-24). "Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells". Nature Biotechnology. 40 (8): 1250–1258. doi:10.1038/s41587-022-01245-x. ISSN 1087-0156. PMC 9376243. PMID 35332339.
- Ghassemi, Saba; Durgin, Joseph S.; Nunez-Cruz, Selene; Patel, Jai; Leferovich, John; Pinzone, Marilia; Shen, Feng; Cummins, Katherine D.; Plesa, Gabriela; Cantu, Vito Adrian; Reddy, Shantan; Bushman, Frederic D.; Gill, Saar I.; O'Doherty, Una; O'Connor, Roddy S. (February 2022). "Rapid manufacturing of non-activated potent CAR T cells". Nature Biomedical Engineering. 6 (2): 118–128. doi:10.1038/s41551-021-00842-6. ISSN 2157-846X. PMC 8860360. PMID 35190680.
- Ledford, Heidi (2023-12-20). "Cancer-fighting CAR-T cells could be made inside body with viral injection". Nature. 625 (7994): 225–226. doi:10.1038/d41586-023-03969-5. PMID 38129613.
- Chakraborty, Samik; Ye, Juan; Wang, Herui; Sun, Mitchell; Zhang, Yaping; Sang, Xueyu; Zhuang, Zhengping (2023-10-23). "Application of toll-like receptors (TLRs) and their agonists in cancer vaccines and immunotherapy". Frontiers in Immunology. 14. doi:10.3389/fimmu.2023.1227833. ISSN 1664-3224. PMC 10626551. PMID 37936697.
- Mikolič, Veronika; Pantović-Žalig, Jelica; Malenšek, Špela; Sever, Matjaž; Lainšček, Duško; Jerala, Roman (June 2024). "Toll-like receptor 4 signaling activation domains promote CAR T cell function against solid tumors". Molecular Therapy: Oncology. 32 (2): 200815. doi:10.1016/j.omton.2024.200815. ISSN 2950-3299. PMC 11152746. PMID 38840781.
- Chen, Xue; Zhang, Yunxiao; Fu, Yao (2022-06-01). "The critical role of Toll-like receptor-mediated signaling in cancer immunotherapy". Medicine in Drug Discovery. 14: 100122. doi:10.1016/j.medidd.2022.100122. ISSN 2590-0986.
- Wu, Ling; Brzostek, Joanna; Sakthi Vale, Previtha Dawn; Wei, Qianru; Koh, Clara K. T.; Ong, June Xu Hui; Wu, Liang-Zhe; Tan, Jia Chi; Chua, Yen Leong; Yap, Jiawei; Song, Yuan; Tan, Vivian Jia Yi; Tan, Triscilla Y. Y.; Lai, Junyun; MacAry, Paul A. (2023-02-21). "CD28-CAR-T cell activation through FYN kinase signaling rather than LCK enhances therapeutic performance". Cell Reports. Medicine. 4 (2): 100917. doi:10.1016/j.xcrm.2023.100917. ISSN 2666-3791. PMC 9975250. PMID 36696897.
- Wang, Enxiu; Wang, Liang-Chuan; Tsai, Ching-Yi; Bhoj, Vijay; Gershenson, Zack; Moon, Edmund; Newick, Kheng; Sun, Jing; Lo, Albert; Baradet, Timothy; Feldman, Michael D.; Barrett, David; Puré, Ellen; Albelda, Steven; Milone, Michael C. (July 2015). "Generation of Potent T-cell Immunotherapy for Cancer using DAP12-based, Multichain, Chimeric Immunoreceptors". Cancer Immunology Research. 3 (7): 815–826. doi:10.1158/2326-6066.CIR-15-0054. ISSN 2326-6066. PMC 4490943. PMID 25941351.
- "First-in-Human Trial to Assess KIR-CAR T-Cell Therapy in MSLN+ Solid Tumors". CGTlive™. 2022-09-25. Retrieved 2024-06-05.
- "152. A Chimeric Antigen Receptor (CARs) Based Upon a Killer Immunoglobulin-Like Receptor (KIR) Triggers Robust Cytotoxic Activity in Solid Tumors". Molecular Therapy. 22: S57. May 2014. doi:10.1016/s1525-0016(16)35165-6. ISSN 1525-0016.
- Xu, Jun; Nunez-Cruz, Selene; Leferovich, John M.; Gulendran, Gayathri; Zhang, Chune; Yucel, Nora D.; Blair, Megan C.; Stanley, William S.; Johnson, Laura A.; Siegel, Don L.; Milone, Michael C. (2024-03-22). "Abstract 6332: Evaluating the relationship of affinity, functional avidity, and in vivo potency in KIR-CAR T cells". Cancer Research. 84 (6_Supplement): 6332. doi:10.1158/1538-7445.AM2024-6332. ISSN 1538-7445.
- Prinzing, Brooke; Schreiner, Patrick; Bell, Matthew; Fan, Yiping; Krenciute, Giedre; Gottschalk, Stephen (2020-11-05). "MyD88/CD40 signaling retains CAR T cells in a less differentiated state". JCI Insight. 5 (21): e136093, 136093. doi:10.1172/jci.insight.136093. ISSN 2379-3708. PMC 7710311. PMID 33148882.
- Collinson-Pautz, Matthew R.; Chang, Wei-Chun; Lu, An; Khalil, Mariam; Crisostomo, Jeannette W.; Lin, Pei-Yi; Mahendravada, Aruna; Shinners, Nicholas P.; Brandt, Mary E.; Zhang, Ming; Duong, MyLinh; Bayle, J. Henri; Slawin, Kevin M.; Spencer, David M.; Foster, Aaron E. (September 2019). "Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies". Leukemia. 33 (9): 2195–2207. doi:10.1038/s41375-019-0417-9. ISSN 1476-5551. PMC 6756044. PMID 30816327.
- "Decision Memo for Chimeric Antigen Receptor (CAR) T-cell Therapy for Cancers (CAG-00451N)". www.cms.gov. Retrieved 2021-03-22.
- "CAR T-cell Therapy: An Update on Coverage and Reimbursement - Hematology.org". www.hematology.org. Archived from the original on 2022-01-24. Retrieved 2021-03-22.
- Fiorenza S, Ritchie DS, Ramsey SD, Turtle CJ, Roth JA (September 2020). "Value and affordability of CAR T-cell therapy in the United States". Bone Marrow Transplantation. 55 (9): 1706–1715. doi:10.1038/s41409-020-0956-8. PMID 32474570. S2CID 218987876.
- Eder M (25 November 2021). "Which countries is CAR T-cell therapy available in? | SingleUseSupport". Retrieved 13 May 2022.
- "Anvisa aprova produto de terapia avançada para tratamento de câncer". Agência Nacional de Vigilância Sanitária - Anvisa (in Brazilian Portuguese). 2022-02-23. Retrieved 2022-06-07.
External links
- CAR T Cells: Engineering Patients' Immune Cells to Treat Their Cancers. National Cancer Institute, July 2019