| |
| Identifiers | |
|---|---|
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.034.060 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CdO4Se |
| Molar mass | 255.381 g·mol |
| Appearance | colourless solid (dihydrate) |
| Density | 3.62 g·cm (dihydrate) |
| Melting point | 100 °C (dihydrate decomposes) |
| Solubility in water | 70.5 g·l |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Warning |
| Hazard statements | H301, H331, H373, H410 |
| Precautionary statements | P260, P261, P264, P270, P271, P273, P301+P316, P304+P340, P316, P319, P321, P330, P391, P403+P233, P405, P501 |
| Related compounds | |
| Other anions | cadmium sulfate cadmium selenite |
| Other cations | zinc selenate mercury selenate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
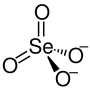
Cadmium selenate is a selenate of cadmium, with the chemical formula CdSeO4.
Preparation
Cadmium selenate can be formed by the reaction of cadmium oxide and selenic acid. The product is a mixture of monohydrate and dihydrate.
- CdO + H2SeO4 → CdSeO4 + H2O
Properties
Cadmium selenate dihydrate is a colorless solid. At 100 °C, the dihydrate releases water of crystallization to form cadmium selenate monohydrate. The monohydrate is monoclinic with space group P21/c (no. 14), while the dihydrate is orthorhombic, with space group Pbca (no. 61).
References
- ^ R. J. Meyer (2013), Cadmium System-Nummer 33, Springer-Verlag, p. 133, ISBN 978-3-662-11295-3
- ^ William M. Haynes (2016), CRC Handbook of Chemistry and Physics, 94th Edition, CRC Press, p. 54, ISBN 978-1-4665-7115-0
- "Cadmium selenate". pubchem.ncbi.nlm.nih.gov.
- ^ Stålhandske, C. (1981-11-15). "Structure of cadmium selenate monohydrate". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 37 (11): 2055–2057. Bibcode:1981AcCrB..37.2055S. doi:10.1107/S0567740881007942.
- H.U.v. Vogel (2013), Chemiker-Kalender, Springer-Verlag, p. 78, ISBN 978-3-662-06237-1
| Cadmium compounds | |
|---|---|
| Cadmium(I) | |
| Cadmium(II) | |
| Salts and covalent derivatives of the selenate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||