A carbon–nitrogen bond is a covalent bond between carbon and nitrogen and is one of the most abundant bonds in organic chemistry and biochemistry.
Nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. Through that pair, nitrogen can form an additional bond to hydrogen making it tetravalent and with a positive charge in ammonium salts. Many nitrogen compounds can thus be potentially basic but its degree depends on the configuration: the nitrogen atom in amides is not basic due to delocalization of the lone pair into a double bond and in pyrrole the lone pair is part of an aromatic sextet.
Similar to carbon–carbon bonds, these bonds can form stable double bonds, as in imines; and triple bonds, such as nitriles. Bond lengths range from 147.9 pm for simple amines to 147.5 pm for C-N= compounds such as nitromethane to 135.2 pm for partial double bonds in pyridine to 115.8 pm for triple bonds as in nitriles.
A CN bond is strongly polarized towards nitrogen (the electronegativities of C and N are 2.55 and 3.04, respectively) and subsequently molecular dipole moments can be high: cyanamide 4.27 D, diazomethane 1.5 D, methyl azide 2.17, pyridine 2.19. For this reason many compounds containing CN bonds are water-soluble. N-philes are group of radical molecules which are specifically attracted to the C=N bonds.
Carbon-nitrogen bond can be analyzed by X-ray photoelectron spectroscopy (XPS). Depending on the bonding states the peak positions differ in N1s XPS spectra.
Nitrogen functional groups
| Chemical class | Bond order | Formula | Structural Formula | Example | Avg. C–N bond length (Å) |
|---|---|---|---|---|---|
| Amines | 1 | R3C-NH2 | 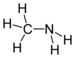 Methylamine |
1.469 (neutral amine) 1.499 (ammonium salt) | |
| Aziridines | 1 | CH2NHCH2 | 
|
 Mitomycin |
1.472 |
| Azides | 1 | R2C-N3 | Phenyl azide |
1.38–1.48 1.47 (methyl azide) 1.432 (phenyl azide) | |
| Anilines | 1 | Ph-NH2 | 
|
 Anisidine |
1.355 (sp N) 1.395 (sp N) 1.465 (ammonium salt) |
| Pyrroles | 1 | 
|
 Porphyrin |
1.372 | |
| Amides | 1.2 | R-CO-NR2 | 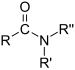
|
 Acetamide |
1.325 (primary) 1.334 (secondary) 1.346 (tertiary) |
| Pyridines | 1.5 | pyr | 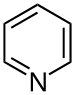
|
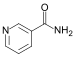 Nicotinamide |
1.337 |
| Imines | 2 | R2C=NR | 
|
 DBN |
1.279 (C=N bond) 1.465 (C–N bond) |
| Nitriles | 3 | R-CN |  Benzonitrile |
1.136 | |
| Isonitriles | 3 | R-NC | TOSMIC |
1.154 |
See also
- Cyanide
- Other carbon bonds with group 15 elements: carbon–nitrogen bonds, carbon–phosphorus bonds
- Other carbon bonds with period 2 elements: carbon–lithium bonds, carbon–beryllium bonds, carbon–boron bonds, carbon–carbon bonds, carbon–nitrogen bonds, carbon–oxygen bonds, carbon–fluorine bonds
- Carbon–hydrogen bond
References
- Organic Chemistry John McMurry 2nd Ed.
- CRC Handbook of Chemistry and Physics 65Th Ed.
- Falzon, Chantal T.; Ryu, Ilhyong; Schiesser, Carl H. (2002). "5-Azahexenoyl radicals cyclize via nucleophilic addition to the acyl carbon rather than 5-exo homolytic addition at the imine". Chemical Communications (20): 2338–9. doi:10.1039/B207729A. PMID 12430429.
- Kato, Tomofumi; Yamada, Yasuhiro; Nishikawa, Yasushi; Otomo, Toshiya; Sato, Hayato; Sato, Satoshi (2021-07-12). "Origins of peaks of graphitic and pyrrolic nitrogen in N1s X-ray photoelectron spectra of carbon materials: quaternary nitrogen, tertiary amine, or secondary amine?". Journal of Materials Science. 56 (28): 15798–15811. doi:10.1007/s10853-021-06283-5. ISSN 1573-4803. S2CID 235793266.
- Yamada, Yasuhiro; Kim, Jungpil; Matsuo, Shintaro; Sato, Satoshi (2014-04-01). "Nitrogen-containing graphene analyzed by X-ray photoelectron spectroscopy". Carbon. 70: 59–74. doi:10.1016/j.carbon.2013.12.061. ISSN 0008-6223.
- Yamada, Yasuhiro; Tanaka, Haruki; Kubo, Shingo; Sato, Satoshi (2021-09-01). "Unveiling Bonding States and Roles of Edges in Nitrogen-Doped Graphene Nanoribbon by X-ray Photoelectron Spectroscopy". Carbon. 185: 342–367. doi:10.1016/j.carbon.2021.08.085. ISSN 0008-6223. S2CID 239687362.
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen. Tables of bond Lengths determined by X-Ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans. II 1987, S1-S19.
- Kumasaki, M.; Kinbara, K.; Wada, Y.; Arai, M.; Tamura, M. (2001). "Azidoacetamide, a neutral small organic azide". Acta Crystallogr. E. 57: o6–o8. doi:10.1107/S160053680001850X.
- Livingston, R. L.; Rao, C. N. Ramachandra (1960). "An Electron Diffraction Investigation of the Molecular Structure of Methyl Azide". J. Phys. Chem. 64 (6): 756–759. doi:10.1021/j100835a012.
- Wagner, Gerald; Arion, Vladimir B.; Brecker, Lothar; Krantz, Carsten; Mieusset, Jean-Luc; Brinker, Udo H. (2009). "Controllable Selective Functionalization of a Cavitand via Solid State Photolysis of an Encapsulated Phenyl Azide". Org. Lett. 11 (14): 3056–3058. doi:10.1021/ol901122h. PMID 19537769.
- Bano, Huma; Yousuf, Sammer (2015). "Crystal structure of p-toluenesulfonylmethyl isocyanide". Acta Crystallogr. E. 71 (6): o412. doi:10.1107/S2056989015008816. PMC 4459310. PMID 26090196. S2CID 26154257.
| Compounds of carbon with other elements in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Legend |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||