| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H16ClCoN4O3 |
| Molar mass | 274.59 g·mol |
| Appearance | red solid |
| Density | 1.79 g/cm |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
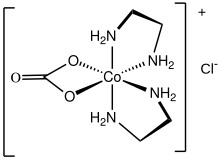
Carbonatobis(ethylenediamine)cobalt(III) chloride is a salt with the formula Cl (en = ethylenediamine). It is a red diamagnetic solid that is soluble in water. It is the monochloride salt of a cationic carbonate complex . The chloride ion in this salt readily undergoes ion exchange. The compound is synthesized by the oxidation of a mixture of cobalt(II) chloride, lithium hydroxide, and ethylenediamine in the presence of carbon dioxide:
- CoCl2 + 2 en + CO2 + 0.5 H2O2 + LiOH → Cl + H2O + LiCl
The cationic complex is octahedral with C2 symmetry.
The carbonato ligand is readily replaced upon acid hydrolysis. Derivatives include the following complexes: cis- and trans-, cis-, cis-, and cis-. Reaction with trifluoromethanesulfonic acid (HOTf) gives OTf.
References
- ^ Springbørg, J.; Schaffer, C. E. (1973). "Dianionobis(ethylenediamine)cobalt(III) Complexes". Inorganic Syntheses. Vol. 14. p. 63-77. doi:10.1002/9780470132456.ch14. ISBN 9780470132456.
- García-Granda, S.; Calvo-Pérez, V.; Gómez-Beltrán, F. (1993). "Structure Redetermination of Carbonatobis(ethlenediamine)cobalt(III) Chloride". Acta Crystallographica Section C Crystal Structure Communications. 49 (2): 322–324. Bibcode:1993AcCrC..49..322G. doi:10.1107/S0108270192006085.
- Dixon, N. E.; Jackson, W. G.; Lawrance, G. A.; Sargeson, A. M. (1983). "Cobalt(III) Amine Complexes with Coordinated Trifluoromethanesulfonate". Inorganic Syntheses. Vol. 22. pp. 103–107. doi:10.1002/9780470132531.ch21. ISBN 978-0-471-88887-1.