| |
| Names | |
|---|---|
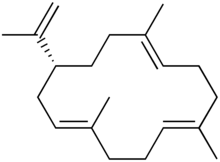
| Preferred IUPAC name (1E,5E,9E,12R)-1,5,9-Trimethyl-12-(prop-1-en-2-yl)cyclotetradeca-1,5,9-triene | |
| Other names
(R,1E,5E,9E)-1,5,9-Trimethyl-12-(prop-1-en-2-yl)cyclotetradeca-1,5,9-triene Neocembrene-A | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H32 |
| Molar mass | 272.47 g/mol |
| Boiling point | 150 to 152 °C (302 to 306 °F; 423 to 425 K) at 0.8 mmHg |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Cembrene A, or sometimes neocembrene, is a natural monocyclic diterpene isolated from corals of the genus Nephthea. It is a colorless oil with a faint wax-like odor.
Cembrene A itself has little importance as a chemical entity, being a trail pheromone for termites; however, the chemical structure of cembrene is central to a very wide variety of other natural products found both in plants and in animals. Pinus leucodermis tree bark and wood essential oils contain a high percentage of cembrene.
Cembrenes are biosynthesized by macrocyclization of geranylgeranyl pyrophosphate.
References
- Vanderah, David J.; Rutledge, Neal; Schmitz, Francis J.; Ciereszko, Leon S (1978). "Marine natural products: cembrene-A and cembrene-C from a soft coral, Nephthea species". Journal of Organic Chemistry. 43 (8): 1614–1616. doi:10.1021/jo00402a040.
- Birch, A. J.; Brown, W. V.; Corrie, J. E. T.; Moore, B. P (1972). "Neocembrene-A, a termite trail pheromone". Journal of the Chemical Society, Perkin Transactions 1. 21: 2653–2658. doi:10.1039/p19720002653.
- ^ Terpenes: Flavors, Fragrances, Pharmaca, Pheromones, Eberhard Breitmaier, page 7. ISBN 978-3-527-31786-8
- Graikou, K.; Gortzi, O.; Mantanis, G.; Chinou, I. (2012). "Chemical composition and biological activity of the essential oil from the wood of Pinus heldreichii Christ. var. leucodermis". European Journal of Wood and Wood Products. 70 (5): 615–620. doi:10.1007/s00107-012-0596-9. ISSN 0018-3768.