| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1-Chloro-1,1,2,2,2-pentafluoroethane | |||
| Other names Freon 115, CFC-115, R-115, Fluorocarbon-115, Genetron 115, Halocarbon 115, Monochloropentafluoroethane | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.854 | ||
| EC Number |
| ||
| E number | E945 (glazing agents, ...) | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1020 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
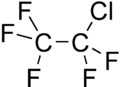
| Chemical formula | C2ClF5 | ||
| Molar mass | 154.466 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | Ethereal | ||
| Melting point | −99 °C (−146 °F; 174 K) | ||
| Boiling point | −39.1 °C (−38.4 °F; 234.1 K) | ||
| Solubility in water | 59 mg/L | ||
| Vapor pressure | 7.9 atm (21°C) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | In high concentrations may cause asphyxiation. | ||
| GHS labelling: | |||
| Pictograms |  
| ||
| Signal word | Warning | ||
| Hazard statements | H420 | ||
| Precautionary statements | P410+P403, P502 | ||
| Flash point | 70.4 °C (158.7 °F; 343.5 K) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | none | ||
| REL (Recommended) | TWA 1000 ppm (6320 mg/m) | ||
| IDLH (Immediate danger) | N.D. | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chloropentafluoroethane is a chlorofluorocarbon (CFC) once used as a refrigerant and also known as R-115 and CFC-115. Its production and consumption has been banned since 1 January 1996 under the Montreal Protocol because of its high ozone depletion potential and very long lifetime when released into the environment. CFC-115 is also a potent greenhouse gas.
Atmospheric properties
The atmospheric abundance of CFC-115 rose from 8.4 parts per trillion (ppt) in year 2010 to 8.7 ppt in 2020 based on analysis of air samples gathered from sites around the world.
| Property | Value |
|---|---|
| Ozone depletion potential (ODP) | 0.44 (CCl3F = 1) |
| Global warming potential (GWP: 100-year) | 5,860 - 7,670 (CO2 = 1) |
| Atmospheric lifetime | 1,020 - 1,700 years |
See also
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0131". National Institute for Occupational Safety and Health (NIOSH).
- http://encyclopedia.airliquide.com/sds/en/030_AL_EN.pdf
- Ozone Depleting Substances List (Montreal Protocol)
- "AGAGE Data and Figures". Massachusetts Institute of Technology. Retrieved 2021-02-11.
- ^ John S. Daniel; Guus J.M. Velders; A.R. Douglass; P.M.D. Forster; D.A. Hauglustaine; I.S.A. Isaksen; L.J.M. Kuijpers; A. McCulloch; T.J. Wallington (2006). "Chapter 8. Halocarbon Scenarios, Ozone Depletion Potentials, and Global Warming Potentials" (PDF). Scientific Assessment of Ozone Depletion: 2006. Geneva, Switzerland: World Meteorological Organization. Retrieved 9 October 2016.
- ^ "Chapter 8". AR5 Climate Change 2013: The Physical Science Basis. p. 731.
- "Refrigerants - Environmental Properties". The Engineering ToolBox. Retrieved 2016-09-12.