
| |
| Names | |
|---|---|
| Preferred IUPAC name 2,4,5,6-Tetrachlorobenzene-1,3-dicarbonitrile | |
| Other names
2,4,5,6-Tetrachloroisophthalonitrile Bravo Daconil Tetrachloroisophthalonitrile Celeste Bronco Agronil Aminil | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.015.990 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3276, 2588 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8Cl4N2 |
| Molar mass | 265.90 g·mol |
| Appearance | white crystalline solid |
| Density | 1.8 g cm, solid |
| Melting point | 250 °C (482 °F; 523 K) |
| Boiling point | 350 °C (662 °F; 623 K) (760 mmHg) |
| Solubility in water | 10 mg/100 mL |
| log P | 2.88–3.86 |
| Hazards | |
| GHS labelling: | |
| Pictograms |     
|
| Signal word | Danger |
| Hazard statements | H317, H318, H330, H335, H351, H410 |
| Precautionary statements | P201, P202, P260, P261, P271, P272, P273, P280, P281, P284, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P312, P320, P321, P333+P313, P363, P391, P403+P233, P405, P501 |
| Related compounds | |
| Related nitriles; organochlorides | benzonitrile; hexachlorobenzene, dichlorobenzene, chlorobenzene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Chlorothalonil (2,4,5,6-tetrachloroisophthalonitrile) is an organic compound mainly used as a broad spectrum, nonsystemic fungicide, with other uses as a wood protectant, pesticide, acaricide, and to control mold, mildew, bacteria, algae. Chlorothalonil-containing products are sold under the names Bravo, Echo, and Daconil. It was first registered for use in the US in 1966. In 1997, it was the third most used fungicide in the US, behind only sulfur and copper, with 12 million pounds (5.4 million kilograms) used in agriculture that year. Including nonagricultural uses, the United States Environmental Protection Agency (EPA) estimates, on average, almost 15 million lb (6.8 million kg) were used annually from 1990 to 1996.
Uses

In the US, chlorothalonil is used predominantly on peanuts (about 34% of usage), potatoes (about 12%), and tomatoes (about 7%), although the EPA recognizes its use on many other crops. It is also used on golf courses and lawns (about 10%) and as a preservative additive in some paints (about 13%), resins, emulsions, and coatings.
Chlorothalonil is commercially available in many different formulations and delivery methods. It is applied as a dust, dry or water-soluble grains, a wettable powder, a liquid spray, a fog, and a dip. It may be applied by hand, by ground sprayer, or by aircraft.
Mechanism of action
Chlorothalonil reacts with glutathione giving an glutathione adduct with elimination of HCl. Its mechanism of action is similar to that of trichloromethyl sulfenyl fungicides such as captan and folpet.
Toxicity
Acute
According to the EPS, chlorothalonil is a toxicity category I eye irritant, producing severe eye irritation. It is in toxicity category II, "moderately toxic", if inhaled (inhaled LD50 0.094 mg/L in rats.) For skin contact and ingestion, chlorothalonil is rated toxicity category IV, "practically nontoxic", meaning the oral and dermal LD50 is greater than 10,000 mg/kg.
Chronic
Long-term exposure to chlorothalonil resulted in kidney damage and tumors in animal tests.
Carcinogenic
International Agency for Research on Cancer IARC has assessed chlorothalonil as a Group 2B "possible human carcinogen", based on observations of cancers and tumors of the kidneys and forestomachs in laboratory animals fed diets containing chlorothalonil.
Environmental
Chlorothalonil was found to be an important factor in the decline of the honey bee population, by making the bees more vulnerable to the gut parasite Nosema ceranae.
Chlorothalonil is highly toxic to fish and aquatic invertebrates, but not toxic to birds.
At a concentration of 164 μg/L, chlorothalonil was found to kill a species of frog within a 24-hour exposure.
Contaminants
Common chlorothalonil synthesis procedures frequently result in contamination of it with small amounts of hexachlorobenzene (HCB), which is toxic. US regulations limit HCB in commercial production to 0.05% of chlorothalonil. According to the EPA report, "post-application exposure to HCB from chlorothalonil is not expected to be a concern based on the low level of HCB in chlorothalonil. 2,3,7,8-Tetrachlorodibenzodioxin being one of the most potent carcinogens known is also a known contaminant".
Environmental contamination
Chlorothalonil has been detected in ambient air Prince Edward Island, as well as in groundwater in Long Island, New York and Florida. In the first three cases, the contamination is presumed to have come from potato farms. It has also been detected in several fish kills in Prince Edward Island.
The main breakdown product of chlorothalonil is hydroxy-2,5,6-trichloro-1,3-dicyanobenzene (SDS-3701). It has been shown to be 30 times more acutely toxic than chlorothalonil and more persistent in the environment. Laboratory experiments have shown it can thin the eggshells of birds, but no evidence supports this happening in the environment.
In 2019, a review of the evidence found that "a high risk to amphibians and fish was identified for all representative uses", and that chlorothalonil breakdown products may cause DNA damage. Chlorothalonil and other fungicides are claimed to be the strongest factor in bumblebee population decline.
- Important metabolites of chlorothalonil
-
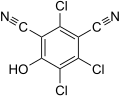 2,4,5-trichloro-6-hydroxyisophthalonitrile (SDS-3701)
2,4,5-trichloro-6-hydroxyisophthalonitrile (SDS-3701)
-
 Chlorothalonil sulfonic acid (R417888)
Chlorothalonil sulfonic acid (R417888)
-
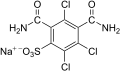 R471811
R471811
Gag order
Syngenta sued to stop communication by Swiss health authorities with the Swiss public regarding the "relevance" of specific metabolites of chlorothalonil that Swiss authorities detected in high concentrations in the groundwater from which hundreds of thousands of Swiss people obtain drinking water. The court banned Swiss health authorities from communicating with the public about the dangers posed by some of the metabolites.
Bans
In March 2019, as a result of the previously mentioned research, the European Union banned the use of chlorothalonil dated to take effect May 20, 2020. Switzerland followed in December 2019.
Production
Chlorothalonil can be produced by the direct chlorination of isophthalonitrile or by dehydration of tetrachloroisophthaloyl amide with phosphoryl chloride. It is a white solid. It breaks down under basic conditions, but is stable in neutral and acidic media. Technical grade chlorothalonil contains traces of dioxins and hexachlorobenzene, a persistent organic pollutant banned under the Stockholm Convention.
References
- "Chlorothalonil". Pubchem.
- ^ Reregistration Eligibility Decision for chlorothalonil, US EPA, 1999.
- PESTICIDE USE IN U.S. CROP PRODUCTION: 1997 Archived 10 December 2006 at the Wayback Machine National Center for Food and Agricultural Policy, 1997.
- Tillman, Ronald; Siegel, Malcolm; Long, John (June 1973), "Mechanism of action and fate of the fungicide chlorothalonil (2,4,5,6-tetrachloroisophthalonitrile) in biological systems: I. Reactions with cells and subcellular components of Saccharomyces pastorianus", Pesticide Biochemistry and Physiology, 3 (2): 160–167, doi:10.1016/0048-3575(73)90100-4
- Siegel, Malcolm R. (1970). "Reactions of certain trichloromethyl sulfenyl fungicides with low-molecular-weight thiols. In vivo studies with cells of Saccharomyces pastorianus". J. Agric. Food Chem. 18 (5): 823–826. doi:10.1021/jf60171a034. PMID 5474238.
- Jeffery S. Pettis, Elinor M. Lichtenberg, Michael Andree, Jennie Stitzinger, Robyn Rose, Dennis vanEngelsdorp "Crop Pollination Exposes Honey Bees to Pesticides Which Alters Their Susceptibility to the Gut Pathogen Nosema ceranae" PLOS One, 24 July 2013, Online: 9 April 2014.
- ^ Environmental Health Criteria 183, World Health Organization, 1996.
- Taegan McMahon, Neal Halstead, Steve Johnson, Thomas R. Raffel, John M. Romansic, Patrick W. Crumrine, Raoul K. Boughton, Lynn B. Martin, Jason R. Rohr "The Fungicide Chlorothalonil is Nonlinearly Associated with Corticosterone Levels, Immunity, and Mortality in Amphibians" Environ Health Perspectives, 2011, Online: 4 April. doi:10.1289/ehp.1002956
- White LM, Ernst WR, Julien G, Garron C, Leger M (2006). "Ambient air concentrations of pesticides used in potato cultivation in Prince Edward Island, Canada". Pest Manag. Sci. 62 (2): 126–36. doi:10.1002/ps.1130. PMID 16358323.
- "Island Fish Kills from 1962 to 2016" (PDF). Government of PEI. Retrieved 12 December 2016.
- Arena, Maria; Auteri, Domenica; Barmaz, Stefania; Bellisai, Giulia; Brancato, Alba; Brocca, Daniela; Bura, Laszlo; Byers, Harry; Chiusolo, Arianna; Court Marques, Daniele; Crivellente, Federica; De Lentdecker, Chloe; Egsmose, Mark; Erdos, Zoltan; Fait, Gabriella; Ferreira, Lucien; Goumenou, Marina; Greco, Luna; Ippolito, Alessio; Istace, Frederique; Jarrah, Samira; Kardassi, Dimitra; Leuschner, Renata; Lythgo, Christopher; Magrans, Jose Oriol; Medina, Paula; Miron, Ileana; Molnar, Tunde; Nougadere, Alexandre; Padovani, Laura; Parra Morte, Juan Manuel; Pedersen, Ragnor; Reich, Hermine; Sacchi, Angela; Santos, Miguel; Serafimova, Rositsa; Sharp, Rachel; Stanek, Alois; Streissl, Franz; Sturma, Juergen; Szentes, Csaba; Tarazona, Jose; Terron, Andrea; Theobald, Anne; Vagenende, Benedicte; Verani, Alessia; Villamar‐Bouza, Laura (January 2018). "Peer review of the pesticide risk assessment of the active substance chlorothalonil". EFSA Journal. 16 (1): e05126. doi:10.2903/j.efsa.2018.5126. PMC 7009683. PMID 32625673.
- Cox, Caroline (1997), "Fungicide Factsheet: Chlorothalonil", Journal of Pesticide Reform, 17 (4): 14–20, archived from the original on 10 November 2010
- ^ Carrington, Damian (29 March 2019). "EU bans UK's most-used pesticide over health and environment fears". The Guardian. Retrieved 29 March 2019.
- "European Commission votes to ban fungicide Chlorothalonil".
- Le Monde, 27 Sept. 2022 "By-Products of Pesticide Banned in 2019 Found in Swiss Drinking Water, Syngenta, The Producer of The Fungicide Chlorothalonil, Has Sued Swiss Health Authorities for Making Public The Danger Posed by Certain Derivatives of The Pesticide,"
- National Farmers' Union of England and Wales. "Chlorothalonil use-by date approaching". NFU Online. Retrieved 16 March 2021.
- "Swiss ban widely-used pesticide over health and environment fears". swissinfo. Retrieved 12 December 2019.
- Franz Müller; Peter Ackermann; Paul Margot. "Fungicides, Agricultural, 2. Individual Fungicides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o12_o06. ISBN 978-3527306732.
External links
- Extension Toxicology Network: Chlorothalonil Pesticide Information Profile
- Chlorothalonil in the Pesticide Properties DataBase (PPDB)
- "2017 Pesticide Use Maps - Low". Water Resources. United States Geological Survey. Retrieved 16 March 2021.
- "2017 Pesticide Use Maps - High". Water Resources. United States Geological Survey. Retrieved 16 March 2021.