| Choroid plexus papilloma | |
|---|---|
| Other names | Papilloma of the choroid plexus |
 | |
| Specialty | Neuro-oncology, Neurosurgery |
Choroid plexus papilloma, also known as papilloma of the choroid plexus, is a rare benign neuroepithelial intraventricular WHO grade I lesion found in the choroid plexus. It leads to increased cerebrospinal fluid production, thus causing increased intracranial pressure and hydrocephalus.
Choroid plexus papilloma occurs in the lateral ventricles of children and in the fourth ventricle of adults. This is unlike most other pediatric tumors and adult tumors, in which the locations of the tumors is reversed. In children, brain tumors are usually found in the infratentorial region and in adults, brain tumors are usually found in the supratentorial space. The relationship is reversed for choroid plexus papillomas.
Epidemiology
CPPs are rare tumors of neuroectodermal origin. They make up 0.4 to 0.6 percent of all intracranial neoplasms in children and are the third most prevalent congenital brain tumors after teratomas and gliomas. With a median age upon diagnosis of 3.5 years, this lesion is often a disease of infancy. They often reside supratentorial in the lateral ventricles of infants (most commonly in the atrium). The fourth ventricle in adults is the optimum location. Adults rarely have it at the cerebellopontine angle.
Etiology
Simian virus (SV) 40 has been linked in studies to the development of choroid plexus tumors (CPTs). The BK and JC viruses have also been linked to the problem. In people with choroid plexus tumors, complexes formed by the big T antigen and the tumor suppressor proteins p53 and pRb have been shown to develop. However, the available evidence do not suggest a causal involvement. There is currently no additional causal component being investigated. One of the most prevalent mutations in CPTs is the TP53 R248W mutation.
Signs and symptoms
Signs of the tumor resulting from increased intracranial pressure are present in 91% of patients, with vomiting, homonymous visual field defects and headache being the most common symptoms. Other symptoms are ear ringing and nausea, loss of balance and mobility, worsening eyesight, memory issues, brain fog, and mood swings.
Pathophysiology
Choroid plexus tumors are divided into three categories by the World Health Organization (2016): papillomas (grade I), atypical tumors (grade II), and carcinomas (grade III). Less than two mitotic figures per 10 high power fields are present in CPPs, two to five are present in atypical ones, and more than five are present in carcinomas. The tumors are visible as pink, soft, spherical lumps with erratic projections and considerable vascularity.
Histopathology
The tumor is neuroectodermal in origin and similar in structure to a normal choroid plexus. They may be created by epithelial cells of the choroid plexus. Papillary fronds lined by bland columnar epithelium are visible under the microscope. Normal absences include mitotic activity, nuclear pleomorphism, and necrosis. Tumors have positive immunohistochemistry for cytokeratin, vimentin, podoplanin, and S-100. Up to 20% of choroid plexus papilloma patients may test positive for glial fibrillary acidic protein (GFAP). Studies have found that fourth ventricle cancers express more S100 than lateral ventricle tumors, and older patients (over 20 years) express more GFAP and transthyretin than younger patients. Some individuals with choroid plexus papilloma have germline TP53 gene mutations, according to genetic analyses. These cancers rarely exhibit nuclear p53 protein positivity. Aicardi syndrome, hypomelanosis of Ito, and 9p duplication are syndromic correlations of choroid plexus papilloma.
-
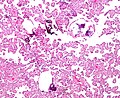 Micrograph of a choroid plexus papilloma. H&E stain.
Micrograph of a choroid plexus papilloma. H&E stain.
-
 Plexuspapillom Detail
Plexuspapillom Detail
-
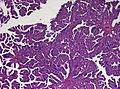 Plexuspapillom Overview
Plexuspapillom Overview
Diagnosis
A neurosonogram via the anterior fontanelle will show an echogenic lesion inside the ventricles if the fontanelles are not united. This lesion exhibits bidirectional flow throughout the diastole, demonstrating blood flow via disorganized vascular arrangement. Sometimes ultrasound scans are used to diagnose the lesions before birth. An isodense or slightly hyperdense lesion inside the ventricles, as well as the resulting ventriculomegaly, are visible on computer tomography (CT). The intraventricular lobulated masses are well-defined and resemble fronds; they are hypointense on T1WI and hyperintense on T2WI on magnetic resonance imaging (MRI). Active blood flow is indicated by the presence of flow voids. A rich vascularity gives the lesions a brilliant enhancing quality. Recent researches have shown that choroid plexus papilloma and choroid plexus cancer may be distinguished from one another using arterial spin labeling.
Treatment
There is disagreement about the best time to do surgery on tumors that were discovered accidentally. An option may be an immediate surgical removal, or surgery might be postponed until follow-up imaging reveals radiographic abnormalities or hydrocephalus, or surgery might be a possibility if the patient starts to experience symptoms. Once hydrocephalus occurs, it is simpler to remove the tumor since there is more room around it and the path to the ventricles is shorter. Such a policy has the drawback that patients may experience focused deficiencies as a result of seizures, subarachnoid hemorrhage, or mass impact. In addition, patients often experience cognitive problems as tumor progress. In symptomatic patients, since these tumors are benign, gross total resection (GTR) is the preferred course of therapy. Because of recent improvements in imaging, surgical techniques, and intensive care quality, the chance of a cure has reached nearly 100% . Significant (12 percent) perioperative mortality occurs in the juvenile population, with catastrophic blood loss accounting for the majority of cases. Preoperative embolization can improve the likelihood of a full tumor resection and reduce this risk. Radiosurgery could be a therapy option. To decrease blood flow and increase tumor resectability, percutaneous stereotactic intratumoral embolization with a sclerosing agent has also been tried. Although it is rarely used, adjuvant chemotherapy can prolong survival and prevent recurrence. Irradiation followed by subtotal resection may be used to treat a developing residual choroid plexus papilloma, making it more likely to success. Malignant tumors and those with leptomeningeal dissemination require adjuvant treatment as well. Bevacizumab is playing a bigger part in disseminated choroid plexus papilloma, according to recent research.
Differential Diagnosis
Choroid plexus papilloma differentials include the following: Meningioma, Chordoid glioma, Rosette-forming glioneuronal tumor, Central nervous system lymphoma Metastasis, Central neurocytoma, Intraventricular tumors such as papillary ependymoma, Subependymoma, Subependymal giant cell tumor, Choroid plexus tumors, Medulloblastoma.
Prognosis
The prognosis of patients has been significantly enhanced by advances in surgical and critical care treatments. Maximum tumor removal is associated with improved progression-free and overall survival. Recurrences are uncommon. Although uncommon, reports of suprasellar metastases and craniospinal seeding have been made.
Complications
Children who have had their intracranial pressure elevated for a long time may come with symptoms such papilledema, optic atrophy, and vision loss that may not improve after surgery. Some may experience postoperative convulsions, bleeding, and cognitive impairments. Additionally, reports of postoperative CSF rhinorrhea have been reported.
References
- McEvoy AW, Harding BN, Phipps KP, Ellison DW, Elsmore AJ, Thompson D, et al. (April 2000). "Management of choroid plexus tumours in children: 20 years experience at a single neurosurgical centre". Pediatric Neurosurgery. 32 (4): 192–199. doi:10.1159/000028933. PMID 10940770.
- Adunka O, Buchman C (11 October 2010). Otology, Neurotology, and Lateral Skull Base Surgery: An Illustrated Handbook. Thieme. pp. 353–. ISBN 978-3-13-149621-8. Retrieved 12 August 2013.
- Bahar M, Hashem H, Tekautz T, Worley S, Tang A, de Blank P, Wolff J. Choroid plexus tumors in adult and pediatric populations: the Cleveland Clinic and University Hospitals experience. J Neurooncol. 2017 May;132(3):427-432.
- ^ Dash C, Moorthy S, Garg K, Singh PK, Kumar A, Gurjar H, Chandra PS, Kale SS. Management of Choroid Plexus Tumors in Infants and Young Children Up to 4 Years of Age: An Institutional Experience. World Neurosurg. 2019 Jan;121:e237-e245.
- Prasad GL, Mahapatra AK. Case series of choroid plexus papilloma in children at uncommon locations and review of the literature. Surg Neurol Int. 2015;6:151.
- Okamoto H, Mineta T, Ueda S, Nakahara Y, Shiraishi T, Tamiya T, Tabuchi K. Detection of JC virus DNA sequences in brain tumors in pediatric patients. J Neurosurg. 2005 Apr;102(3 Suppl):294-8.
- ^ Zhen HN, Zhang X, Bu XY, Zhang ZW, Huang WJ, Zhang P, Liang JW, Wang XL. Expression of the simian virus 40 large tumor antigen (Tag) and formation of Tag-p53 and Tag-pRb complexes in human brain tumors. Cancer. 1999 Nov 15;86(10):2124-32.
- Yankelevich M, Finlay JL, Gorsi H, Kupsky W, Boue DR, Koschmann CJ, Kumar-Sinha C, Mody R. Molecular insights into malignant progression of atypical choroid plexus papilloma. Cold Spring Harb Mol Case Stud. 2021 Feb;7(1)
- Ruggeri L, Alberio N, Alessandrello R, Cinquemani G, Gambadoro C, Lipani R, Maugeri R, Nobile F, Iacopino DG, Urrico G, Battaglia R. Rapid malignant progression of an intraparenchymal choroid plexus papillomas. Surg Neurol Int. 2018;9:131.
- Khade S, Shenoy A. Ectopic Choroid Plexus Papilloma. Asian J Neurosurg. 2018 Jan-Mar;13(1):191-194.
- Ikota H, Tanaka Y, Yokoo H, Nakazato Y. Clinicopathological and immunohistochemical study of 20 choroid plexus tumors: their histological diversity and the expression of markers useful for differentiation from metastatic cancer. Brain Tumor Pathol. 2011 Jul;28(3):215-21
- Prendergast N, Goldstein JD, Beier AD. Choroid plexus adenoma in a child: expanding the clinical and pathological spectrum. J Neurosurg Pediatr. 2018 Apr;21(4):428-433.
- Paulus W, Jänisch W. Clinicopathologic correlations in epithelial choroid plexus neoplasms: a study of 52 cases. Acta Neuropathol. 1990;80(6):635-41.
- Tabori U, Shlien A, Baskin B, Levitt S, Ray P, Alon N, Hawkins C, Bouffet E, Pienkowska M, Lafay-Cousin L, Gozali A, Zhukova N, Shane L, Gonzalez I, Finlay J, Malkin D. TP53 alterations determine clinical subgroups and survival of patients with choroid plexus tumors. J Clin Oncol. 2010 Apr 20;28(12):1995-2001.
- Li Y, Chetty S, Feldstein VA, Glenn OA. Bilateral Choroid Plexus Papillomas Diagnosed by Prenatal Ultrasound and MRI. Cureus. 2021 Mar 06;13(3):e13737.
- Cao LR, Chen J, Zhang RP, Hu XL, Fang YL, Cai CQ. Choroid Plexus Papilloma of Bilateral Lateral Ventricle in an Infant Conceived by in vitro Fertilization. Pediatr Neurosurg. 2018;53(6):401-406.
- Pandey SK, Mani SE, Sudhakar SV, Panwar J, Joseph BV, Rajshekhar V. Reliability of Imaging-Based Diagnosis of Lateral Ventricular Masses in Children. World Neurosurg. 2019 Jan 17
- Dangouloff-Ros V, Grevent D, Pagès M, Blauwblomme T, Calmon R, Elie C, Puget S, Sainte-Rose C, Brunelle F, Varlet P, Boddaert N. Choroid Plexus Neoplasms: Toward a Distinction between Carcinoma and Papilloma Using Arterial Spin-Labeling. AJNR Am J Neuroradiol. 2015 Sep;36(9):1786-90.
- ^ Laarakker AS, Nakhla J, Kobets A, Abbott R. Incidental choroid plexus papilloma in a child: A difficult decision. Surg Neurol Int. 2017;8:86.
- Ito H, Nakahara Y, Kawashima M, Masuoka J, Abe T, Matsushima T. Typical Symptoms of Normal-Pressure Hydrocephalus Caused by Choroid Plexus Papilloma in the Cerebellopontine Angle. World Neurosurg. 2017 Feb;98:875.e13-875.e17.
- Toescu SM, James G, Phipps K, Jeelani O, Thompson D, Hayward R, Aquilina K. Intracranial Neoplasms in the First Year of Life: Results of a Third Cohort of Patients From a Single Institution. Neurosurgery. 2019 Mar 01;84(3):636-646.
- Aljared T, Farmer JP, Tampieri D. Feasibility and value of preoperative embolization of a congenital choroid plexus tumour in the premature infant: An illustrative case report with technical details. Interv Neuroradiol. 2016 Dec;22(6):732-735.
- Kim IY, Niranjan A, Kondziolka D, Flickinger JC, Lunsford LD. Gamma knife radiosurgery for treatment resistant choroid plexus papillomas. J Neurooncol. 2008 Oct;90(1):105-10.
- Jung GS, Ruschel LG, Leal AG, Ramina R. Embolization of a giant hypervascularized choroid plexus papilloma with onyx by direct puncture: a case report. Childs Nerv Syst. 2016 Apr;32(4):717-21.
- Turkoglu E, Kertmen H, Sanli AM, Onder E, Gunaydin A, Gurses L, Ergun BR, Sekerci Z. Clinical outcome of adult choroid plexus tumors: retrospective analysis of a single institute. Acta Neurochir (Wien). 2014 Aug;156(8):1461-8; discussion 1467-8.
- Morshed RA, Lau D, Sun PP, Ostling LR. Spinal drop metastasis from a benign fourth ventricular choroid plexus papilloma in a pediatric patient: case report. J Neurosurg Pediatr. 2017 Nov;20(5):471-479.
- Anderson MD, Theeler BJ, Penas-Prado M, Groves MD, Yung WK. Bevacizumab use in disseminated choroid plexus papilloma. J Neurooncol. 2013 Sep;114(2):251-3.
- Muly S, Liu S, Lee R, Nicolaou S, Rojas R, Khosa F. MRI of intracranial intraventricular lesions. Clin Imaging. 2018 Nov - Dec;52:226-239
- Siegfried A, Morin S, Munzer C, Delisle MB, Gambart M, Puget S, Maurage CA, Miquel C, Dufour C, Leblond P, André N, Branger DF, Kanold J, Kemeny JL, Icher C, Vital A, Coste EU, Bertozzi AI. A French retrospective study on clinical outcome in 102 choroid plexus tumors in children. J Neurooncol. 2017 Oct;135(1):151-160.
- Safaee M, Oh MC, Sughrue ME, Delance AR, Bloch O, Sun M, Kaur G, Molinaro AM, Parsa AT. The relative patient benefit of gross total resection in adult choroid plexus papillomas. J Clin Neurosci. 2013 Jun;20(6):808-12
- Abdulkader MM, Mansour NH, Van Gompel JJ, Bosh GA, Dropcho EJ, Bonnin JM, Cohen-Gadol AA. Disseminated choroid plexus papillomas in adults: A case series and review of the literature. J Clin Neurosci. 2016 Oct;32:148-54.
- Fujimura M, Onuma T, Kameyama M, Motohashi O, Kon H, Yamamoto K, Ishii K, Tominaga T. Hydrocephalus due to cerebrospinal fluid overproduction by bilateral choroid plexus papillomas. Childs Nerv Syst. 2004 Jul;20(7):485-8.
- Ward C, Phipps K, de Sousa C, Butler S, Gumley D. Treatment factors associated with outcomes in children less than 3 years of age with CNS tumours. Childs Nerv Syst. 2009 Jun;25(6):663-8.
- Lechanoine F, Zemmoura I, Velut S. Treating Cerebrospinal Fluid Rhinorrhea without Dura Repair: A Case Report of Posterior Fossa Choroid Plexus Papilloma and Review of the Literature. World Neurosurg. 2017 Dec;108:990.e1-990.e9.
External links
| Classification | D |
|---|---|
| External resources |
![]() Media related to Choroid plexus papilloma at Wikimedia Commons
Media related to Choroid plexus papilloma at Wikimedia Commons
- Choroid Plexus Papilloma MRI, CT, and pathology images from MedPix
| Tumours of the nervous system | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocrine |
| ||||||||||||||||||||||
| CNS |
| ||||||||||||||||||||||
| PNS: | |||||||||||||||||||||||
| Other | |||||||||||||||||||||||
| Note: Not all brain tumors are of nervous tissue, and not all nervous tissue tumors are in the brain (see brain metastasis). | |||||||||||||||||||||||
| Diseases of the nervous system, primarily CNS | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation |
| ||||||||||||||||||||||||
| Brain/ encephalopathy |
| ||||||||||||||||||||||||
| Both/either |
| ||||||||||||||||||||||||