 Chromium, Cr Oxygen, O | |

| |
| Names | |
|---|---|
| IUPAC name Chromium trioxide | |
| Other names Chromic anhydride, Chromium(VI) oxide, Chromic acid (misnomer) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.189 |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1463 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CrO3 |
| Molar mass | 99.993 g·mol |
| Appearance | Dark red granular solid, deliquescent |
| Odor | Odorless |
| Density | 2.7 g/cm (20 °C) |
| Melting point | 197 °C (387 °F; 470 K) |
| Boiling point | 250 °C (482 °F; 523 K) decomposes |
| Solubility in water |
|
| Solubility | Soluble in H2SO4, HNO3, (CH3CH2)2O, CH3COOH, (CH3)2CO |
| Magnetic susceptibility (χ) | +40·10 cm/mol |
| Thermochemistry | |
| Std molar entropy (S298) |
73.2 J/(mol·K) |
| Std enthalpy of formation (ΔfH298) |
−589.3 kJ/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |     
|
| Signal word | Danger |
| Hazard statements | H271, H301+H311, H314, H317, H330, H334, H335, H340, H350, H361f, H372, H410 |
| Precautionary statements | P210, P260, P280, P303+P361+P353, P304+P340+P310, P305+P351+P338 |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 80 mg/kg (rats, oral) |
| Safety data sheet (SDS) | ICSC 1194 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.
Production, structure, and basic reactions
Chromium trioxide is generated by treating sodium dichromate with sulfuric acid:
- H2SO4 + Na2Cr2O7 → 2 CrO3 + Na2SO4 + H2O
Approximately 100,000 tonnes are produced annually by this or similar routes.
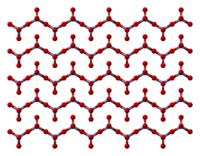
The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center therefore shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3.

The structure of monomeric CrO3 has been calculated using density functional theory, and is predicted to be pyramidal (point group C3v) rather than planar (point group D3h).
Chromium trioxide decomposes above 197 °C, liberating oxygen and eventually giving Cr2O3:
- 4 CrO3 → 2 Cr2O3 + 3 O2
It is used in organic synthesis as an oxidant, often as a solution in acetic acid, or acetone in the case of the Jones oxidation. In these oxidations, the Cr(VI) converts primary alcohols to the corresponding carboxylic acids and secondary alcohols to ketones. The reactions are shown below:
- Primary alcohols to carboxylic acids
- 4 CrO3 + 3 RCH2OH + 12 H → 3 RCOOH + 4 Cr + 9 H2O
- Secondary alcohols to ketones
- 2 CrO3 + 3 R2CHOH + 6 H → 3 R2C=O + 2 Cr + 6 H2O
Applications
Chromium trioxide is mainly used in chrome plating. It is typically employed with additives that affect the plating process but do not react with the trioxide. The trioxide reacts with cadmium, zinc, and other metals to generate passivating chromate films that resist corrosion. It is also used in the production of synthetic rubies. Chromic acid solution is also used in applying types of anodic coating to aluminium, which are primarily used in aerospace applications. On the International Space Station, it is used to control bacteria growth in the wastewater storage tank. A chromic acid/phosphoric acid solution is also the preferred stripping agent of anodic coatings of all types.
Safety
Chromium trioxide is highly toxic, corrosive, and carcinogenic. It is the main example of hexavalent chromium, an environmental hazard. The related chromium(III) derivatives are not particularly dangerous; thus, reductants are used to destroy chromium(VI) samples.
Chromium trioxide, being a powerful oxidizer, will ignite organic materials such as alcohols on contact.
Images
-
 A concentrated solution of potassium dichromate in water.
A concentrated solution of potassium dichromate in water.
-
 Addition of sulfuric acid to the solution.
Addition of sulfuric acid to the solution.
-
 Crystallization of chromium trioxide from the reaction.
Crystallization of chromium trioxide from the reaction.
References
- ^ Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). D. Van Nostrand Company. p. 250.
- "chromium(VI) oxide". chemister.ru.
- Pradyot, Patnaik (2003). Handbook of Inorganic Chemicals. The McGraw-Hill Companies, Inc. ISBN 0-07-049439-8.
- ^ Sigma-Aldrich Co., Chromium(VI) oxide. Retrieved on 2021-11-22.
- ^ "Chromium trioxide". chemicalland21.com. AroKor Holdings Inc. Retrieved 2014-06-15.
- ^ Anger, G.; Halstenberg, J.; Hochgeschwender, K.; Scherhag, C.; Korallus, U.; Knopf, H.; Schmidt, P.; Ohlinger, M. (2000). "Chromium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a07_067. ISBN 3527306730.
- Mamyrbaev, Arstan Abdramanovich; Dzharkenov, Timur Agataevich; Imangazina, Zina Amangalievna; Satybaldieva, Umit Abulkhairovna (2015-04-16). "Mutagenic and carcinogenic actions of chromium and its compounds". Environmental Health and Preventive Medicine. 20 (3). Springer Science and Business Media LLC: 159–167. doi:10.1007/s12199-015-0458-2. ISSN 1342-078X. PMC 4434237. PMID 25877777.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- Stephens, J. S.; Cruickshank, D. W. J. (1970). "The crystal structure of (CrO3)∞". Acta Crystallographica Section B. 26 (3): 222. doi:10.1107/S0567740870002182.
- Zhai, H. J.; Li, S.; Dixon, D. A.; Wang, L. S. (2008). "Probing the Electronic and Structural Properties of Chromium Oxide Clusters (CrO
3)
n and (CrO3)n (n = 1–5): Photoelectron Spectroscopy and Density Functional Calculations". Journal of the American Chemical Society. 130 (15): 5167–77. doi:10.1021/ja077984d. PMID 18327905. - "Chromium Trioxide (MSDS)". J. T. Baker. Archived from the original on 2015-01-12. Retrieved 2007-09-13.
- The environmental impact of hexavalent chromium inspired the 2000 biographical Hollywood movie Erin Brockovich.
External links
- ATSDR Case Studies in Environmental Medicine: Chromium Toxicity U.S. Department of Health and Human Services
- Chromium Trioxide at The Periodic Table of Videos (University of Nottingham)
- Reactions with Chromium Trioxide as Oxidizing Agent
| Chromium compounds | |||
|---|---|---|---|
| Chromium(0) |
| ||
| Chromium(I) |
| ||
| Chromium(II) |
| ||
| Chromium(II, III) | |||
| Chromium(III) | |||
| Chromium(IV) | |||
| Chromium(V) | |||
| Chromium(VI) |
| ||
| Polyatomic ion | |||



