 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
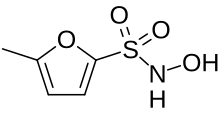
| Formula | C5H7NO4S |
| Molar mass | 177.17 g·mol |
| 3D model (JSmol) | |
SMILES
| |
Cimlanod (development codes CXL-1427 and BMS-986231) is an experimental drug for the treatment of acute decompensated heart failure. It was discovered by Cardioxyl Pharmaceuticals, which was acquired by Bristol-Myers Squibb. It is a precursor of nitroxyl.
Cimlanod is a prodrug of CXL-1020.
A preliminary study showed efficacy in patients with class III and IV heart failure. A phase II clinical trial was completed in 2016.
References
- "BMS 986231". AdisInsight. Springer Nature Switzerland AG. Retrieved 2017-05-22.
Alternative Names: BMS-986231; CXL 1427; HNO Donor
- "CXL 1020". AdisInsight. Springer Nature Switzerland AG. Retrieved 2017-05-22.
- Zoler ML (22 May 2016). "Nitroxyl prodrug shows promise in acute heart failure".
- Clinical trial number NCT02157506 for "A Dose Ranging Phase IIa Study of 6 Hour Intravenous Dosages of CXL-1427 in Patients Hospitalized With Heart Failure" at ClinicalTrials.gov
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |