| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name But-2-ene | |||
| Other names β-Butylene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) |
| ||
| Beilstein Reference | 1718755 1361341 | ||
| ChEBI |
| ||
| ChemSpider | |||
| ECHA InfoCard | 100.003.140 | ||
| EC Number |
| ||
| Gmelin Reference | 25196 1140 1141 | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII |
| ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C4H8 | ||
| Molar mass | 56.106 g/mol | ||
| Density | 0.641 g/ml (cis, 3.7 °C) 0.626 g/ml (trans, 0.9 °C) | ||
| Melting point | −138.9 °C (−218.0 °F; 134.2 K) (cis) -105.5 °C (trans) | ||
| Boiling point | 0.8 to 3.7 °C (33.4 to 38.7 °F; 273.9 to 276.8 K) (Z = 3.7 °C) (E = 0.8 °C) | ||
| Magnetic susceptibility (χ) |
| ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |  
| ||
| Signal word | Danger | ||
| Hazard statements | H220 | ||
| Precautionary statements | P210, P377, P381, P403 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | −72 °C (−98 °F; 201 K) | ||
| Autoignition temperature |
325 °C (617 °F; 598 K) | ||
| Related compounds | |||
| Related butenes | 1-Butene cis-2-Butene trans-2-Butene Isobutene | ||
| Related compounds | Butane Butyne | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
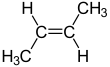
But-2-ene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism (also known as (E/Z)-isomerism); that is, it exists as two geometric isomers cis-but-2-ene ((Z)-but-2-ene) and trans-but-2-ene ((E)-but-2-ene).
It is a petrochemical, produced by the catalytic cracking of crude oil or the dimerization of ethylene. Its main uses are in the production of high-octane gasoline (petrol) on alkylation units and butadiene, although some but-2-ene is also used to produce the solvent butanone via hydration reaction to butan-2-ol followed by oxidation.
The two isomers are extremely difficult to separate by distillation because of the proximity of their boiling points (~4 °C for cis and ~1 °C for trans). However, separation is unnecessary in most industrial settings, as both isomers behave similarly in most of the desired reactions. A typical industrial but-2-ene mixture is 70% (Z)-but-2-ene (cis-isomer) and 30% (E)-but-2-ene (trans-isomer). Butane and but-1-ene are common impurities, present at 1% or more in industrial mixtures, which also contain smaller amounts of isobutene, butadiene and butyne.
References
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- cis-2-Butene, International Chemical Safety Card 0397, Geneva: International Programme on Chemical Safety, March 1996. trans-2-Butene, International Chemical Safety Card 0398, Geneva: International Programme on Chemical Safety, March 1996.
- ^ 2-Butene (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, February 1995.
- Chemical Safety Information from Intergovernmental Organizations Archived December 9, 2009, at the Wayback Machine
External links
- SIDS Initial Assessment Report for 2-Butene from the Organisation for Economic Co-operation and Development (OECD)