| |
| Names | |
|---|---|
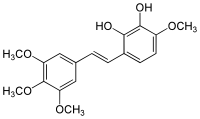
| Preferred IUPAC name 3-Methoxy-6-benzene-1,2-diol | |
| Other names Combretastatin A1; OXi4500 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C18H20O6 |
| Molar mass | 332.352 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Combretastatin A-1 is a combretastatin and a stilbenoid. It can be found in Combretum afrum, the Eastern Cape South African Bushwillow tree.
Biological effects in mammals
It is an antiangiogenic agent acting by destabilizing tubulin, which induces cell apoptosis of proliferating endothelial cells.
Derivatives as drugs
Currently designated an orphan drug by the FDA, combretastatin A1 diphosphate (OXi4503 or CA1P) is in Phase I clinical trials for relapsed and refractory acute myeloid leukemia and myelodysplastic syndrome.
References
- ^ Pettit, G. R.; Singh, S. B.; Niven, M. L.; Hamel, E.; Schmidt, J. M. (1987). "Isolation, Structure, and Synthesis of Combretastatins A-1 and B-1, Potent New Inhibitors of Microtubule Assembly, Derived from Combretum caffrum". Journal of Natural Products. 50 (1): 119–131. doi:10.1021/np50049a016. PMID 3598594.
- "A Phase I Clinical Trial of OXi4503 for Relapsed and Refractory Acute Myelogenous Leukemia (AML) and Myelodysplastic Syndromes (MDS)". 7 August 2017.
| Hydroxystilbenes and their glycosides (monomeric forms) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dihydroxylated | |||||||||
| Trihydroxylated | |||||||||
| Tetrahydroxylated | |||||||||
| O-methylated |
| ||||||||
| carboxylated | |||||||||
| other acylations | |||||||||
| Glycosides |
| ||||||||
| Oligomeric forms | oligostilbenoids | ||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |