| |
| Names | |
|---|---|
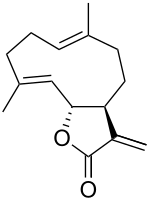
| Preferred IUPAC name (3aS,6E,10E,11aR)-6,10-Dimethyl-3-methylidene-3a,4,5,8,9,11a-hexahydrocyclodecafuran-2(3H)-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.208.663 |
| MeSH | (+)-Costunolide |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H20O2 |
| Molar mass | 232.323 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
(+)-Costunolide is a naturally occurring sesquiterpene lactone, first isolated in Saussurea costus roots in 1960. It is also found in lettuce.
Synthesis
It is synthesized through the mevalonate pathway, seen in Figure 1. The synthesis begins with the cyclization of compound 1, farnesyl pyrophosphate (FPP), which is mediated by a sesquiterpene cyclase, (+)-germacrene A synthase, to form compound 2, (+)-germacryl cation. Inside this same enzyme, a proton is lost to form 3, (+)-germacrene A. The isoprenyl side chain of (+)-germacrene A is then hydroxylated by (+)-germacrene A hydroxylase, which is a cytochrome P450 enzyme, to form 4. NAD(P) dependent hydrogenase(s) then oxidize 4, germacra-1(10),4,11(13)-trien-12-ol, through the intermediate 5, germacra-1(10),4,11(13)-trien-12-al to form compound 6, germacrene acid. The cytochrome P450 enzyme, (+)-costunolide synthase, which is a NADPH and O2 dependent enzyme, then oxidizes germacrene acid to give the alcohol intermediate, 7, which then cyclizes to form the lactone 8, (+)-costunolide.

Figure 1. Biosynthesis of (+)-Costunolide.
References
- ^ Kraker, J.; Franssen, M.; Dalm, M.; Groot, A.; Bouwmeester, H. (April 2001). "Biosynthesis of Germacrene A Carboxylic Acid in Chicory Roots. Demonstration of a Cytochrome P450 (+)-Germacrene A Hydroxylase and NADP+-Dependent Sesquiterpenoid Dehydrogenase(s) Involved in Sesquiterpene Lactone Biosynthesis". Plant Physiology. 125 (4): 1930–1940. doi:10.1104/pp.125.4.1930. PMC 88848. PMID 11299372.
- ^ Dewick, Paul M. (2009). Medicinal Natural Products: A Biosynthetic Approach. West Sussex, United Kingdom: John Wiley & Sons Ltd. p. 214.
- Kraker, J.; Franssen, M.; Joerink, M.; Groot, A.; Bouwmeester, H. (April 2002). "Biosynthesis of Costunolide, Dihydrocostunolide, and Leucodin. Demonstration of Cytochrome P450-Catalyzed Formation of the Lactone Ring Present in Sesquiterpene Lactones of Chicory". Plant Physiology. 129: 257–258. doi:10.1104/pp.010957. PMC 155889. PMID 12011356.