| |
| Names | |
|---|---|
| IUPAC name methanol | |
| Preferred IUPAC name (cyclohexane-1,4-diyl)dimethanol | |
| Other names 1,4–Cyclohexanedimethanol; CHDM; 1,4-Bis(hydroxymethyl)cyclohexane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.002.972 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H16O2 |
| Molar mass | 144.21 g/mol |
| Appearance | White waxy solid |
| Density | 1.02 g/ml |
| Melting point | 41 to 61 °C (106 to 142 °F; 314 to 334 K) |
| Boiling point | 284 to 288 °C (543 to 550 °F; 557 to 561 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
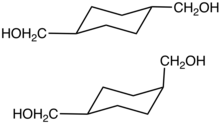
Cyclohexanedimethanol (CHDM) is a mixture of isomeric organic compounds with formula C6H10(CH2OH)2. It is a colorless low-melting solid used in the production of polyester resins. Commercial samples consist of a mixture of cis and trans isomers. It is a di-substituted derivative of cyclohexane and is classified as a diol, meaning that it has two OH functional groups. Commercial CHDM typically has a cis/trans ratio of 30:70.
Production
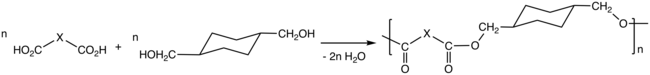
CHDM is produced by catalytic hydrogenation of dimethyl terephthalate (DMT). The reaction conducted in two steps beginning with the conversion of DMT to the diester dimethyl 1,4-cyclohexanedicarboxylate (DMCD):
- C6H4(CO2CH3)2 + 3 H2 → C6H10(CO2CH3)2
In the second step DMCD is further hydrogenated to CHDM:
- C6H10(CO2CH3)2 + 4 H2 → C6H10(CH2OH)2 + 2 CH3OH
A copper chromite catalyst is usually used industrially. The cis/trans ratio of the CHDM is affected by the catalyst.
Byproduct of this process are 4-methylcyclohexanemethanol (CH3C6H10CH2OH) and the monoester methyl 4-methyl-4-cyclohexanecarboxylate (CH3C6H10CO2CH3, CAS registry number 51181-40-9). The leading producers in CHDM are Eastman Chemical in US and SK Chemicals in South Korea.
Applications
Via the process called polycondensation, CHDM is a precursor to polyesters. It is one of the most important comonomers for production of polyethylene terephthalate (PET), or polyethylene terephthalic ester (PETE), from which plastic bottles are made. In addition it maybe spun to form carpet fibers.
Thermoplastic polyesters containing CHDM exhibit enhanced strength, clarity, and solvent resistance. The properties of the polyesters vary from the high melting crystalline poly(1,4-cyclohexylenedimethylene terephthalate), PCT, to the non-crystalline copolyesters derived from both ethylene glycol and CHDM. The properties of these polyesters also is affected by the cis/trans ratio of the CHDM monomer. CHDM reduces the degree of crystallinity of PET homopolymer, improving its processability. The copolymer tends to resist degradation, e.g. to acetaldehyde. The copolymer with PET is known as glycol-modified polyethylene terephthalate, PETG. PETG is used in many fields, including electronics, automobiles, barrier, and medical, etc.
CHDM is a raw material for the production of 1,4-cyclohexanedimethanol diglycidyl ether, an epoxy diluent. The key use for this diglycidyl ether is to reduce the viscosity of epoxy resins.
References
- S.R. Turner; Y. Li (2010). "Synthesis and Properties of Cyclic Diester Based Aliphatic Copolyesters". Journal of Polymer Science Part A: Polymer Chemistry. 48 (10): 2162–2169. doi:10.1002/pola.23985.
- J. M. Thomas; R. Raja (2002). "The materials Chemistry of Inorganic Catalyst". Australian Journal of Chemistry. 54: 551–560. doi:10.1071/CH01150.
- Peter Werle, Marcus Morawietz, Stefan Lundmark, Kent Sörensen, Esko Karvinen and Juha Lehtonen "Alcohols, Polyhydric" Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_305.pub2
- S.R. Turner (2004). "Development of amorphous copolyesters based on 1,4- cyclohexane-dimethanol". Journal of Polymer Science Part A: Polymer Chemistry. 42 (23): 5847–5852. doi:10.1002/pola.20460.
- S. Andjelic; D.D. Jamiolkowski; R. Bezwada (2007). "Mini-review The Polyoxaesters". Polymer International. 56: 1063–1077. doi:10.1002/pi.2257.
- Hatton. "Nylon vs. Polyester Carpet Fibers: Comparison Guide". Homedit. Retrieved 2023-08-17.
- S. R. Turner; R.W. Seymour; T.W. Smith (2001). "Cyclohexanedimethanol Polyesters". Encyclopedia of Polymer Science and Technology. doi:10.1002/0471440264.pst257. ISBN 0471440264.
- Crivello, James V. (2006). "Design and synthesis of multifunctional glycidyl ethers that undergo frontal polymerization". Journal of Polymer Science Part A: Polymer Chemistry. 44 (21): 6435–6448. Bibcode:2006JPoSA..44.6435C. doi:10.1002/pola.21761. ISSN 0887-624X.
- Monte, Salvatore J. (1998), Pritchard, Geoffrey (ed.), "Diluents and viscosity modifiers for epoxy resins", Plastics Additives: An A-Z reference, Polymer Science and Technology Series, vol. 1, Dordrecht: Springer Netherlands, pp. 211–216, doi:10.1007/978-94-011-5862-6_24, ISBN 978-94-011-5862-6, archived from the original on 2022-04-11, retrieved 2022-03-29