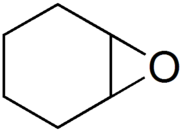
| |
| Names | |
|---|---|
| IUPAC name 7-Oxabicycloheptane | |
| Other names Epoxycyclohexane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.005.462 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H10O |
| Molar mass | 98.145 g·mol |
| Appearance | Colorless liquid |
| Density | 0.97 g·cm |
| Melting point | ca. -40 °C |
| Boiling point | ca. 130 °C |
| Solubility in water | Practically insoluble |
| Vapor pressure | 12 mbar (at 20 °C) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Cyclohexene oxide is a cycloaliphatic epoxide. It can react in cationic polymerization to poly(cyclohexene oxide). As cyclohexene is monovalent, poly(cyclohexene oxide) is a thermoplastic.
Production
Cyclohexene oxide is produced in epoxidation reaction from cyclohexene. The epoxidation can take place either in a homogeneous reaction by peracids or heterogeneous catalysis (e.g. silver and molecular oxygen).
In industrial production the heterogeneously catalyzed synthesis is preferred because of better atom economy, a simpler separation of the product and easier recycling of catalyst. A short overview and an investigation of the oxidation of cyclohexene by hydrogen peroxide is given in the literature. In recent times the catalytic oxidation of cyclohexene by (immobilized) metalloporphyrin complexes has been found to be an efficient way.
In laboratory, cyclohexene oxide can also be prepared by reacting cyclohexene with magnesium monoperoxyphthalate (MMPP) in a mixture of isopropanol and water as solvent at room temperature.
With this method, good yields up to 85 % can be reached.
Properties and reactions
Cyclohexene oxide has been studied extensively by analytical methods. Cyclohexene oxide can be polymerized in solution, catalyzed by a solid acid catalyst.
References
- ^ Record of Epoxycyclohexane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 1 February 2014.
- M. Quenard; V. Bonmarin; G. Gelbard (1987). "Epoxidation of olefins by hydrogen peroxide catalyzed by phosphonotungstic complexes". Tetrahedron Letters. 28 (20): 2237–2238. doi:10.1016/S0040-4039(00)96089-1.
- Ha Q. Pham; Maurice J. Marks (2005), "Epoxy Resins", Ullmann's Encyclopedia of Industrial Chemistry (in German), doi:10.1002/14356007.a09_547.pub2, ISBN 3527306730
- Siegfried Rebsdat; Dieter Mayer (2001), "Ethylene Oxide", Ullmann's Encyclopedia of Industrial Chemistry (in German), doi:10.1002/14356007.a10_117, ISBN 3527306730
- Morazzoni, Franca; Canevali, Carmen; d'Aprile, Fiorenza; Bianchi, Claudia L.; Tempesti, Ezio; Giuffrè, Luigi; Airoldi, Giuseppe (1995). "Spectroscopic investigation of the molybdenum active sites on Mo heterogeneous catalysts for alkene epoxidation". Journal of the Chemical Society, Faraday Transactions. 91 (21): 3969–3974. doi:10.1039/FT9959103969.
- Ambili, V K; Dr.Sugunan, S (April 2011), Faculty of Sciences (ed.), Studies on Catalysis by Ordered Mesoporous SBA-15 Materials Modified with Transition Metals (in German), Cochin University of Science and Technology, retrieved 2014-07-27
{{citation}}: CS1 maint: multiple names: authors list (link) - Costa, Andréia A. Ghesti; Grace F. de Macedo; Julio L. Braga; Valdeilson S. Santos; Marcello M. Dias; José A. Dias; Sílvia C.L. (2008). "Immobilization of Fe, Mn and Co tetraphenylporphyrin complexes in MCM-41 and their catalytic activity in cyclohexene oxidation reaction by hydrogen peroxide". Journal of Molecular Catalysis A: Chemical. 282 (1–2): 149–157. doi:10.1016/j.molcata.2007.12.024.
- Xian-Tai Zhou; Hong-Bing Ji; Jian-Chang Xu; Li-Xia Pei; Le-Fu Wang; Xing-Dong Yao (2007). "Enzymatic-like mediated olefins epoxidation by molecular oxygen under mild conditions". Tetrahedron Letters. 48 (15): 2691–2695. doi:10.1016/j.tetlet.2007.02.066.
- Brougham, Paul; Cooper, Mark S.; Cummerson, David A.; Heaney, Harry; Thompson, Nicola (1987). "Oxidation Reactions Using Magnesium Monoperphthalate: A Comparison with m-Chloroperoxybenzoic Acid". Synthesis. 1987 (11): 1015–1017. doi:10.1055/s-1987-28153. Retrieved 2020-07-31.
- RM Ibberson; O. Yamamuro; I. Tsukushi (2006). "The crystal structures and phase behaviour of cyclohexene oxide". Chemical Physics Letters. 423 (4–6): 454–458. Bibcode:2006CPL...423..454I. doi:10.1016/j.cplett.2006.04.004.
- Ahmed Yahiaoui; Mohammed Belbachir; Jeanne Claude Soutif; Laurent Fontaine (2005). "Synthesis and structural analyses of poly(1,2-cyclohexene oxide) over solid acid catalyst". Materials Letters. 59 (7): 759–767. doi:10.1016/j.matlet.2004.11.017.

