| It has been suggested that this article be merged with Sodium cyclopentadienide. (Discuss) Proposed since November 2024. |
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Cyclopentadienide | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | or Cp | ||
| Molar mass | 65.09 g/mol | ||
| Conjugate acid | Cyclopentadiene | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of
and abbreviated as Cp. It is formed by the deprotonation of cyclopentadiene. The cyclopentadienyl anion is a ligand which binds to a metal in organometallic chemistry.
The first salt with this anion, potassium cyclopentadienide, was prepared by Johannes Thiele in 1901 but there wasn't much interest in the topic until the discovery of ferrocene in the 1950s.
Resonance and aromaticity
See also: Sodium cyclopentadienide Resonance structures of the cyclopentadienyl anion
Resonance structures of the cyclopentadienyl anion
The cyclopentadienyl anion is a planar, cyclic, regular-pentagonal ion; it has 6 π-electrons (4n + 2, where n = 1), which fulfills Hückel's rule of aromaticity. Each double bond and lone pair provides 2 π-electrons, which are delocalized into the ring.
Cyclopentadiene has a pKa of about 16. It is acidic relative to many carbon acids. The enhanced acidity is attributed to stabilization of the conjugate base, cyclopentadienyl anion.
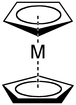
Ligand
Main article: Cyclopentadienyl complexCyclopentadienyl anions form a variety of cyclopentadienyl complexes.
See also
References
- "Cyclopentadienide". PubChem Compound Database. National Center for Biotechnology Information. Retrieved 14 April 2016.
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 66, ISBN 978-0-471-72091-1
- Johannes Thiele (January 1901). "Ueber Abkömmlinge des Cyclopentadiëns". Berichte der Deutschen Chemischen Gesellschaft (in German). 34 (1): 68–71. doi:10.1002/CBER.19010340114. ISSN 0365-9496. Wikidata Q126217369.
- Paul, Satadal; Goswami, Tamal; Misra, Anirban (2015-10-01). "Noncomparative scaling of aromaticity through electron itinerancy". AIP Advances. 5 (10): 107211. Bibcode:2015AIPA....5j7211P. doi:10.1063/1.4933191.
- C. Elschenbroich (2006). Organometallics. VCH. ISBN 978-3-527-29390-2.

