| cystathionine gamma-synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Cystathionine gamma-synthase homotetramer, Helicobacter pylori Cystathionine gamma-synthase homotetramer, Helicobacter pylori | |||||||||
| Identifiers | |||||||||
| EC no. | 2.5.1.48 | ||||||||
| CAS no. | 9030-70-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
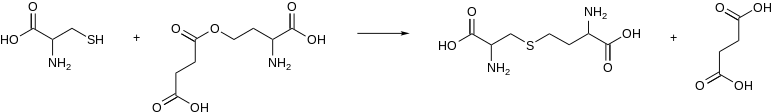
In enzymology, a cystathionine gamma-synthase (EC 2.5.1.48) is an enzyme that catalyzes the formation of cystathionine from cysteine and an activated derivative of homoserine, e.g.:
In microorganisms, the activated substrate of this enzyme is O4-succinyl-L-homoserine or O4-acetyl-L-homoserine. Cystathionine gamma-synthase from plants uses L-homoserine phosphate instead.
This enzyme belongs to the family of transferases, specifically those transferring aryl or alkyl groups other than methyl groups. The systematic name of this enzyme class is O4-succinyl-L-homoserine:L-cysteine S-(3-amino-3-carboxypropyl)transferase. Other names in common use include O-succinyl-L-homoserine succinate-lyase (adding cysteine), O-succinylhomoserine (thiol)-lyase, homoserine O-transsuccinylase, O-succinylhomoserine synthase, O-succinylhomoserine synthetase, cystathionine synthase, cystathionine synthetase, homoserine transsuccinylase, 4-O-succinyl-L-homoserine:L-cysteine, and S-(3-amino-3-carboxypropyl)transferase. This enzyme participates in 4 metabolic pathways: methionine metabolism, cysteine metabolism, selenoamino acid metabolism, and sulfur metabolism. It employs one cofactor, pyridoxal phosphate.
References
- Steegborn C, Laber B, Messerschmidt A, Huber R, Clausen T (August 2001). "Crystal structures of cystathionine gamma-synthase inhibitor complexes rationalize the increased affinity of a novel inhibitor". Journal of Molecular Biology. 311 (4): 789–801. doi:10.1006/jmbi.2001.4880. PMID 11518531.
- Flavin M, Slaughter C (March 1967). "Enzymatic synthesis of homocysteine or methionine directly from O-succinyl-homoserine". Biochimica et Biophysica Acta. 132 (2): 400–5. doi:10.1016/0005-2744(67)90158-1. PMID 5340123.
- Kaplan MM, Flavin M (October 1966). "Cystathionine gamma-synthetase of Salmonella. Catalytic properties of a new enzyme in bacterial methionine biosynthesis". The Journal of Biological Chemistry. 241 (19): 4463–71. doi:10.1016/S0021-9258(18)99743-7. PMID 5922970.
- Wiebers JL, Garner HR (January 1967). "Homocysteine and cysteine synthetases of Neurospora crassa. Purification, properties, and feedback control of activity". The Journal of Biological Chemistry. 242 (1): 12–23. doi:10.1016/S0021-9258(18)96312-X. PMID 6016326.
- Wiebers JL, Garner HR (December 1967). "Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast, and Escherichia coli". The Journal of Biological Chemistry. 242 (23): 5644–9. doi:10.1016/S0021-9258(18)99405-6. PMID 12325384.
- Clausen T, Huber R, Prade L, Wahl MC, Messerschmidt A (December 1998). "Crystal structure of Escherichia coli cystathionine gamma-synthase at 1.5 A resolution". The EMBO Journal. 17 (23): 6827–38. doi:10.1093/emboj/17.23.6827. PMC 1171030. PMID 9843488.
- Ravanel S, Gakière B, Job D, Douce R (April 1998). "Cystathionine gamma-synthase from Arabidopsis thaliana: purification and biochemical characterization of the recombinant enzyme overexpressed in Escherichia coli". The Biochemical Journal. 331 ( Pt 2) (2): 639–48. doi:10.1042/bj3310639. PMC 1219399. PMID 9531508.
| Enzymes | |
|---|---|
| Activity | |
| Regulation | |
| Classification | |
| Kinetics | |
| Types |
|
This EC 2.5 enzyme-related article is a stub. You can help Misplaced Pages by expanding it. |
 L-cystathionine + succinate
L-cystathionine + succinate