| ACKR1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ACKR1, atypical chemokine receptor 1 (Duffy blood group), CCBP1, CD234, Dfy, FY, GPD, GpFy, WBCQ1, DARC, DARC/ACKR1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 613665; MGI: 1097689; HomoloGene: 48067; GeneCards: ACKR1; OMA:ACKR1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Duffy antigen/chemokine receptor (DARC), also known as Fy glycoprotein (FY) or CD234 (Cluster of Differentiation 234), is a protein that in humans is encoded by the ACKR1 gene.
The Duffy antigen is located on the surface of red blood cells, and is named after the patient in whom it was discovered. The protein encoded by this gene is a glycosylated membrane protein and a non-specific receptor for several chemokines. The protein is also the receptor for the human malarial parasites Plasmodium vivax, Plasmodium knowlesi and simian malarial parasite Plasmodium cynomolgi. Polymorphisms in this gene are the basis of the Duffy blood group system.
History
It was noted in the 1920s that black Africans had some intrinsic resistance to malaria, but the basis for this remained unknown. The Duffy antigen gene was the fourth gene associated with the resistance after the genes responsible for sickle cell anaemia, thalassemia and glucose-6-phosphate dehydrogenase.
In 1950, the Duffy antigen was discovered in a multiply-transfused hemophiliac named Richard Duffy, whose serum contained the first example of anti-Fya antibody. In 1951, the antibody to a second antigen, Fyb, was discovered in serum. Using these two antibodies, three common phenotypes were defined: Fy(a+b+), Fy(a+b-), and Fy(a-b+).
Several other types were later discovered bringing the current total up to 6: Fya, Fyb, Fy3, Fy4, Fy5 and Fy6. Only Fya, Fyb and Fy3 are considered clinically important. Reactions to Fy5 have also rarely been reported. The Fy4 antigen, originally described on Fy (a–b–) RBCs, is now thought to be a distinct, unrelated antigen and is no longer included in the FY system.
Genetics and genomics
The Duffy antigen/chemokine receptor gene (gp-Fy; CD234) is located on the long arm of chromosome 1 (1.q22-1.q23) and was cloned in 1993. The gene was first localised to chromosome 1 in 1968, and was the first blood system antigen to be localised. It is a single copy gene spanning over 1500 bases and is in two exons. The gene encodes a 336 amino acid acidic glycoprotein. It carries the antigenic determinants of the Duffy blood group system which consist of four codominant alleles—FY*A and FY*B—coding for the Fy-a and Fy-b antigens respectively, FY*X and FY*Fy, five phenotypes (Fy-a, Fy-b, Fy-o, Fy-x and Fy-y) and five antigens. Fy-x is a form of Fy-b where the Fy-b gene is poorly expressed. Fy-x is also known as Fy-b or Fy-b.
This gene has been redesignated ACKR1.
Fy-a and Fy-b differ by in a single amino acid at position 42: glycine in Fy-a and aspartic acid in Fy-b (guanine in Fy-a and adenosine in Fy-b at position 125). A second mutation causing a Duffy negative phenotype is known: the responsible mutation is G -> A at position 298. The genetic basis for the Fy(a-b-) phenotype is a point mutation in the erythroid specific promoter (a T -> C mutation at position -33 in the GATA box). This mutation occurs in the Fy-b allele and has been designated Fy-b (erythroid silent). Two isotypes have been identified. The Fy-x allele is characterized by a weak anti-Fy-b reaction and appears to be the result of two separate transitions: Cytosine265Threonine (Arginine89Cysteine) and Guanine298Adenosine (Alanine100Threonine). A third mutation (a transversion) in this gene has also been described - G145T (Alanine49Serine) - that has been associated with the Fy-x phenotype.
Most Duffy negative black people carry a silent Fy-b allele with a single T to C substitution at nucleotide -33, impairing the promoter activity in erythroid cells by disrupting a binding site for the GATA1 erythroid transcription factor. The gene is still transcribed in non erythroid cells in the presence of this mutation.
The Duffy negative phenotype occurs at low frequency among whites (~3.5%) and is due to a third mutation that results in an unstable protein (Arg89Cys: cytosine -> thymidine at position 265).
The silent allele has evolved at least twice in the black population of Africa and evidence for selection for this allele has been found. The selection pressure involved here appears to be more complex than many text books might suggest. An independent evolution of this phenotype occurred in Papua New Guinea has also been documented.
A comparative study of this gene in seven mammalian species revealed significant differences between species. The species examined included Pan troglodytes (chimpanzee), Macaca mulatta (rhesus monkey), Pongo pygmaeus (orangutan), Rattus norvegicus (brown rat), Mus musculus (mouse), Monodelphis domestica (opossum), Bos taurus (cow) and Canis familiaris (dog).
Three exons are present in humans and chimpanzees, whereas only two exons occur in the other species. This additional exon is located at the 5' end and is entirely non coding. Both intron and exon size vary considerably between the species examined. Between the chimpanzee and the human, 24 differences in the nucleotide sequence were noted. Of these 18 occurred in non coding regions. Of the remaining 6, 3 were synonymous and 3 non synonymous mutations. The significance of these mutations if any is not known.
The mouse ortholog has been cloned and exhibits 63% homology to the human gene at the amino acid level. The mouse gene is located on chromosome 1 between the genetic markers Xmv41 and D1Mit166. The mouse gene has two exons (100 and 1064 nucleotides in length), separated by a 461 base pair intron. In the mouse DARC is expressed during embryonic development between days 9.5 and 12.
In yellow baboons (Papio cynocephalus) mutations in this gene have been associated with protection from infection with species of the genus Hepatocystis.
The ancestral form of extant DARC alleles in humans appears to be the FY*B allele.
The gene appears to be under strong purifying selection. The cause of this selective pressure has not yet been identified.
Molecular biology
Biochemical analysis of the Duffy antigen has shown that it has a high content of α-helical secondary structure - typical of chemokine receptors. Its N-glycans are mostly of the triantennary complex type terminated with α2-3- and α2-6-linked sialic acid residues with bisecting GlcNAc and α1-6-linked fucose at the core.
The Duffy antigen is expressed in greater quantities on reticulocytes than on mature erythrocytes. While the Duffy antigen is expressed on bone marrow erythroblasts and circulating erythrocytes it is also found on Purkinje cells of the cerebellum, endothelial cells of thyroid capillaries, the post-capillary venules of some organs including the spleen, liver and kidney and the large pulmonary venules. Duffy antigen has then a very unique cell expression profile in cerebellar neurons, venular endothelial cells and erythroid cells. In some people who lack the Duffy antigen on their erythrocytes it is still expressed in the other cell types.
It has two potential N-linked glycosylation sites at asparagine (Asn) 16 and Asn27.
The Duffy antigen has been found to act as a multispecific receptor for chemokines of both the C-C and C-X-C families, including:
- monocyte chemotactic protein-1 (MCP-1) - CCL2
- regulated upon activation normal T expressed and secreted (RANTES) - CCL5
- melanoma growth stimulatory activity (MSGA-α), KC, neutrophil-activating protein 3 (NAP-3) - CXCL1/CXCL2
and the angiogenic CXC chemokines:
- Growth related gene alpha (GRO-α) - CXCL1
- Platelet factor 4 - CXCL4
- ENA-78 - CXCL5
- Neutrophil activating peptide-2 (NAP-2) - CXCL7
- Interleukin-8 (IL-8) - CXCL8
Consequently, the Fy protein is also known as DARC (Duffy Antigen Receptor for Chemokines). The chemokine binding site on the receptor appears to be localised to the amino terminus. The antigen is predicted to have 7 transmembrane domains, an exocellular N-terminal domain and an endocellular C-terminal domain. Alignment with other seven transmembrane G-protein-coupled receptors shows that DARC lacks the highly conserved DRY motif in the second intracellular loop of the protein that is known to be associated with G-protein signaling. Consistent with this finding ligand binding by DARC does not induce G-protein coupled signal transduction nor a Ca2+ flux unlike other chemokine receptors. Based on these alignments the Duffy antigen is considered to be most similar to the interleukin-8B receptors.
Scatchard analysis of competition binding studies has shown high affinity binding to the Duffy antigen with dissociation constants (KD) binding values of 24 ± 4.9, 20 ± 4.7, 41.9 ± 12.8, and 33.9 ± 7 nanoMoles for MGSA, interleukin-8, RANTES and monocyte chemotactic peptide-1 respectively.
In DARC-transfected cells, DARC is internalized following ligand binding and this led to the hypothesis that expression of DARC on the surface of erythrocytes, endothelial, neuronal cells and epithelial cells may act as a sponge and provide a mechanism by which inflammatory chemokines may be removed from circulation as well as their concentration modified in the local environment. This hypothesis has also been questioned after knock out mice were created. These animals appeared healthy and had normal responses to infection. While the function of the Duffy antigen remains presently (2006) unknown, evidence is accumulating that suggests a role in neutrophil migration from the blood into the tissues and in modulating the inflammatory response.
The protein is also known to interact with the protein KAI1 (CD82) a surface glycoprotein of leukocytes and may have a role in the control of cancer.
The Duffy antigen has been shown to exist as a constitutive homo-oligomer and that it hetero-oligomerizes with the CC chemokine receptor CCR5 (CD195). The formation of this heterodimer impairs chemotaxis and calcium flux through CCR5, whereas internalization of CCR5 in response to ligand binding remains unchanged.
DARC has been shown to internalise chemokines but does not scavenge them. It mediates chemokine transcytosis, which leads to apical retention of intact chemokines and more leukocyte migration.
Binding melanoma growth-stimulating activity inhibits the binding of P. knowlesi to DARC.
Population genetics
Differences in the racial distribution of the Duffy antigens were discovered in 1954, when it was found that the overwhelming majority of people of African descent had the erythrocyte phenotype Fy(a-b-): 68% in African Americans and 88-100% in African people (including more than 90% of West African people). This phenotype is exceedingly rare in Whites. Because the Duffy antigen is uncommon in those of Black African descent, the presence of this antigen has been used to detect genetic admixture. In a sample of unrelated African Americans (n = 235), Afro-Caribbeans (n = 90) and Colombians (n = 93), the frequency of the -46T (Duffy positive) allele was 21.7%, 12.2% and 74.7% respectively.
Overall the frequencies of Fya and Fyb antigens in Whites are 66% and 83% respectively, in Asians 99% and 18.5% respectively and in blacks 10% and 23% respectively. The frequency of Fy3 is 100% Whites, 99.9% Asians and 32% Blacks. Phenotype frequencies are:
- Fy(a+b+): 49% Whites, 1% Blacks, 9% Chinese
- Fy(a-b+): 34% Whites, 22% Blacks, <1% Chinese
- Fy(a+b-): 17% Whites, 9% Blacks, 91% Chinese
While a possible role in the protection of humans from malaria had been previously suggested, this was only confirmed clinically in 1976. Since then many surveys have been carried out to elucidate the prevalence of Duffy antigen alleles in different populations including:
- The mutation Ala100Thr (G -> A in the first codon position—base number 298) within the FY*B allele was thought to be purely a White genotype, but has since been described in Brazilians. However, the study's authors point out that the Brazilian population has arisen from intermarriage between Portuguese, Black Africans, and Indians, which accounts for the presence of this mutation in a few members of Brazil's non-White groups. Two of the three Afro-Brazilian test subjects that were found to have the mutation (out of a total of 25 Afro-Brazilians tested) were also related to one another, as one was a mother and the other her daughter.
- This antigen along with other blood group antigens was used to identify the Basque people as a genetically separate group. Its use in forensic science is under consideration.
- The Andaman and Nicobar Islands, part of India, were originally inhabited by 14 aboriginal tribes. Several of these have gone extinct. One surviving tribe—the Jarawas—live in three jungle areas of South Andaman and one jungle area in Middle Andaman. The area is endemic for malaria. The causative species is Plasmodium falciparum: there is no evidence for the presence of Plasmodium vivax. Blood grouping revealed an absence of both Fy(a) and Fy(b) antigens in two areas and a low prevalence in two others.
- In the Yemenite Jews the frequency of the Fy allele is 0.5879. The frequency of this allele varies from 0.1083 to 0.2191 among Jews from the Middle East, North Africa and Southern Europe. The incidence of Fya among Ashkenazi Jews is 0.44 and among the non-Ashkenazi Jews it is 0.33. The incidence of Fyb is higher in both groups with frequencies of 0.53 and 0.64 respectively.
- In the Chinese ethnic populations—the Han and the She people—the frequencies of Fya and Fyb alleles were 0.94 and 0.06 and 0.98 and 0.02 respectively.
- The frequency of the Fya allele in most Asian populations is ~95%.
- In Grande Comore (also known as Ngazidja) the frequency of the Fy(a- b-) phenotype is 0.86.
- The incidence of Fy(a+b-) in northern India among blood donors is 43.85%.
- In the Maghreb, Horn of Africa and the Nile Valley, the Afroasiatic (Hamitic-Semitic) speaking populations are largely Duffy-positive. Between 70%-98% of Hamito-Semitic groups in Ethiopia were found to be Duffy-positive. Serological and DNA based analysis of 115 unrelated Tunisians also found an FY*X frequency of 0.0174; FY*1 = 0.291 (expressed 0.260, silent 0.031); FY*2 = 0.709 (expressed 0.427; silent 0.282). Since the FY*2 silent is the most common allele in West Africa, its minor occurrence in the sample probably represents recent diffusion from the latter region.
- In Nouakchott, Mauritania overall 27% of the population are Duffy-positive. 54% of Moors are Duffy antigen positive, while only 2% of black ethnic groups (mainly Poular, Soninke and Wolof) are Duffy positive.
- A map of the Duffy antigen distribution has been produced. The most prevalent allele globally is FY*A. Across sub-Saharan Africa the predominant allele is the silent FY*B variant.
- In Iran the Fy (a-b-) phenotype was found in 3.4%.
There appears to have been a selective sweep in Africa which reduced the incidence of this antigen there. This sweep appears to have occurred between 6,500 and 97,200 years ago (95% confidence interval)
The distribution within India has been studied in some detail.
Clinical significance
Historically the role of this antigen other than its importance as a receptor for Plasmodium protozoa has not been appreciated. Recent work has identified a number of additional roles for this protein.
Malaria
On erythrocytes, the Duffy antigen acts as a receptor for invasion by the human malarial parasites P. vivax and P. knowlesi. This was first shown in 1980. Duffy negative individuals whose erythrocytes do not express the receptor are believed to be resistant to merozoite invasion although P. vivax infection has been reported in Duffy negative children in Kenya, suggesting a role in resistance to disease, not infection. This antigen may also play a role in erythrocyte invasion in the rodent malarial parasite P. yoelii. The epitope Fy6 is required for P. vivax invasion.
The protection to P. vivax malaria conferred by the absence of the Duffy antigen appears to be very limited at best in Madagascar. Although 72% of the population are Duffy antigen negative, 8.8% of the Duffy antigen negative individuals were asymptomatic carriers of P. vivax. Malaria has also been found in Angola and Equatorial Guinea in Duffy negative individuals. P. vivax malaria in a Duffy antigen negative individual in Mauritania has also been reported. Similar infections have been reported in Brazil and Kenya. Additional cases of infection in Duffy antigen negative individuals have been reported from the Congo and Uganda. A study in Brazil of the protection against P. vivax offered by the lack of the Duffy antigen found no differential resistance to malaria vivax between Duffy antigen positive and negative individuals.
Nancy Ma's night monkey (A. nancymaae) is used as an animal model of P. vivax infection. This species' erythrocytes possess the Duffy antigen and this antigen is used as the receptor for P. vivax on the erythrocytes in this species.
Examination of this gene in 497 patients in the Amazonas State, Brazil, made by the doctor Sérgio Albuquerque, suggests that the genotypes FY*A/FY*B-33 and FY*B/FY*B-33 (where -33 refers to the null mutation at position -33 in the GATA box) may have an advantage over the genotypes FY*A/FY*B and FY*A/FY*A, FY*A/FY*B, FY*A/FY*X and FY*B/FY*X. FY*A/FY*B and FY*A/FY*A genotypes showed to be associated with increased rates of P. vivax infection and FY*B/FY*X and FY*A/FY*X were shown to be associated with the low levels of parasitism.
A difference between the susceptibility to Plasmodium vivax malaria has been reported. Erythrocytes expressing Fya had 41-50% lower binding of P. vivax compared with Fyb cells. Individuals with the Fy(a+b-) phenotype have a 30-80% reduced risk of clinical vivax but not falciparum malaria.
The binding of platelet factor 4 (CXCL4) appears to be critical for the platelet induced killing of P. falciparum.
The Duffy antigen binding protein in P. vivax is composed of three subdomains and is thought to function as a dimer. The critical DARC binding residues are concentrated at the dimer interface and along a relatively flat surface spanning portions of two subdomains.
A study in Brazil confirmed the protective effect of FY*A/FY*O against malaria. In contrast the genotype FY*B/FY*O was associated with a greater risk.
Asthma
Asthma is more common and tends to be more severe in those of African descent. There appears to be a correlation with both total IgE levels and asthma and mutations in the Duffy antigen.
Hematopoiesis
Duffy antigen plays a fundamental role on hematopoiesis. Indeed, nucleated red blood cells present in the bone marrow have high expression of DARC, which facilitates their direct contact with hematopoietic stem cells. The absence of erythroid DARC alters hematopoiesis including stem and progenitor cells, which ultimately gives rise to phenotypically distinct neutrophils. As a result, mature neutrophils of Duffy-negative individuals carry more molecular "weapons" against infectious pathogens. Therefore, alternative physiological patterns of hematopoiesis and bone marrow cell outputs depend on the expression of DARC in the erythroid lineage.
Benign ethnic neutropenia
Individuals with the Duffy-null genotype have a persistently lower neutrophil count than the typical laboratory normal range, but the lower amount of circulating neutrophils associated with this genotype does not seem to confer an increased risk of infection. Clinical use of the term "benign ethnic neutropenic" to describe this phenomenon remains widespread, but the term is problematic as the Duffy-null genotype is common in individuals with African and certain Middle Eastern ancestries, and the term implies that individuals with European ancestry have the normal reference neutrophil count. The term "Duffy-null associated neutrophil count" (DANC) has been proposed as a replacement.
The distinctive neutrophils that are formed in the absence of DARC on erythroid lineage (see above - role of DARC on hematopoiesis) readily leave the blood stream, which explains the apparent lower numbers of neutrophils in the blood of Duffy-null individuals. Failure to recognize that individuals with African ancestry often have healthy Duffy-null antigen-associated neutrophil counts instead of neutropenia has historically contributed to inequity in access to medications that require blood monitoring due to risk of neutropenia, including chemotherapy and the antipsychotic medication clozapine. The lower number of circulating neutrophils can cause individuals with the Duffy-null genotype to fall below what typically would be considered safe to continue these treatments, despite new data showing that neutrophil functioning is preserved in these individuals.
Cancer
Interactions between the metastasis suppressor KAI1 on tumor cells and the cytokine receptor DARC on adjacent vascular cells suppresses tumor metastasis. In human breast cancer samples low expression of the DARC protein is significantly associated with estrogen receptor status, both lymph node and distant metastasis and poor survival.
Endotoxin response
The procoagulant response to lipopolysaccharide (bacterial endotoxin) is reduced in Duffy antigen negative Africans compared with Duffy positive Whites. This difference is likely to involve additional genes.
HIV infection
A connection has been found between HIV susceptibility and the expression of the Duffy antigen. The absence of the DARC receptor appears to increase the susceptibility to infection by HIV. However once established, the absence of the DARC receptor appears to slow down the progression of the disease.
HIV-1 appears to be able to attach to erythrocytes via DARC.
The association between the Duffy antigen and HIV infection appears to be complex. Leukopenia (a low total white cell count) is associated with relatively poor survival in HIV infection and this association is more marked in whites than in people of Black African descent, despite the (on average) lower white cell counts found in black Africans. This difference appears to correlate with a particular genotype (-46C/C) associated with the absence of the Duffy antigen. This genotype has only been found in black Africans and their descendants. The strength of this association increases inversely with the total white cell count. The basis for this association is probably related to the role of the Duffy antigen in cytokine binding but this has yet to be verified.
A study of 142 black South African high-risk female sex workers over 2 years revealed a seroconversion rate of 19.0%. Risk of seroconversion appeared to be correlated with Duffy-null-associated low neutrophil counts.
Inflammation
An association with the levels monocyte chemoattractant protein-1 has been reported.
In the Sardinian population, an association of several variants in the DARC gene (coding and non-coding) correlates with increased serum levels of monocyte chemoattractant protein (MCP -1). A new variant in this population, consisting of the amino acid substitution of arginine for a cysteine at position 89 of the protein diminishes the ability to bind chemokines.
DARC has also been linked to rheumatoid arthritis (RA), possibly displaying chemokines such as CXCL5 on the surface of endothelial cells within the synovium, increasing the recruitment of neutrophils in the disease state.
Lung transplantation
The Duffy antigen has been implicated in lung transplantation rejection.
Multiple myeloma
An increased incidence of Duffy antigen has been reported in patients with multiple myeloma compared with healthy controls.
Pneumonia
The Duffy antigen is present in the normal pulmonary vascular bed. Its expression is increased in the vascular beds and alveolar septa of the lung parenchyma during suppurative pneumonia.
Pregnancy
Duffy antigen has been implicated in haemolytic disease of the newborn.
Prostate cancer
Experimental work has suggested that DARC expression inhibits prostate tumor growth. Men of black African descent are at greater risk of prostate cancer than are men of either European or Asian descendant (60% greater incidence and double the mortality compared to Whites). However, the contribution of DARC to this increased risk has been tested in Jamaican males of black African descent. It was found that none of the increased risk could be attributed to the DARC gene. The reason for this increased risk is as yet unknown.
Renal transplantion
Antibodies and a cellular response to the Duffy antigen have been associated with renal transplant rejection.
Sickle cell anaemia
Duffy antigen-negative individuals with sickle cell anaemia tend to sustain more severe organ damage than do those with the Duffy antigen. Duffy-positive patients exhibit higher counts of white blood cells, polynuclear neutrophils, higher plasma levels of IL-8 and RANTES than Duffy-negative patients.
Southeast Asian ovalocytosis
There is a ~10% increase in Fy expression in Southeast Asian ovalocytosis erythrocytes.
Transfusion medicine
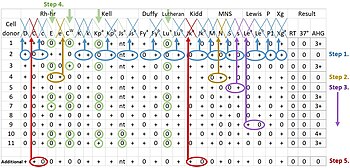
A Duffy negative blood recipient may have a transfusion reaction if the donor is Duffy positive. Since most Duffy-negative people are of African descent, blood donations from people of black African origin are important to transfusion banks.
Transfusion data
International Society for Blood Transfusion (ISBT) symbol: FY
ISBT number: 008
Gene symbol: FY
Gene name: Duffy blood group
Number of Duffy antigens: 6
Antibody type
Almost entirely IgG. IgG1 usually predominates. IgM does occur but is rare.
Antibody behavior
Anti-Fy is a common antibody while anti-Fy is approximately 20 times less common., They are reactive at body temperature and are therefore clinically significant, although they do not typically bind complement. Antibodies are acquired through exposure (pregnancy or history of blood transfusion) and subsequent alloimmunization. They display dosage (react more strongly to homozygous cells versus heterozygous cells).
Transfusion reactions
Typically mild but may be serious, even fatal. Although these usually occur immediately they may occur after a delay (up to 24 hours). These reactions are usually caused by anti-Fy or anti-Fy. anti-Fy3 may cause acute or delayed hemolytic transfusion reactions, but only rarely. Anti-Fy5 may also cause delayed hemolytic transfusion reactions.
Hemolytic disease of the fetus and newborn
Hemolytic disease of the fetus and newborn is typically mild but rarely may be serious. Almost always due to anti-Fy and rarely anti-Fyb or Fy3.
References
- ^ GRCh38: Ensembl release 89: ENSG00000213088 – Ensembl, May 2017
- ^ GRCm38: Ensembl release 89: ENSMUSG00000037872 – Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "ACKR1 gene". U.S. National Library of Medicine Genetics Home Reference. August 17, 2020. Retrieved September 9, 2020.
- ^ Chaudhuri A, Polyakova J, Zbrzezna V, Williams K, Gulati S, Pogo AO (November 1993). "Cloning of glycoprotein D cDNA, which encodes the major subunit of the Duffy blood group system and the receptor for the Plasmodium vivax malaria parasite". Proc. Natl. Acad. Sci. U.S.A. 90 (22): 10793–7. Bibcode:1993PNAS...9010793C. doi:10.1073/pnas.90.22.10793. PMC 47864. PMID 8248172.
- Tournamille C, Colin Y, Cartron JP, Le Van Kim C (June 1995). "Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals". Nat. Genet. 10 (2): 224–8. doi:10.1038/ng0695-224. PMID 7663520. S2CID 7125832.
- Kosaisavee V, Suwanarusk R, Chua AC, Kyle DE, Malleret B, Zhang R, Imwong M, Imerbsin R, Ubalee R, Sámano-Sánchez H, Yeung BK, Ong JJ, Lombardini E, Nosten F, Tan KS, Bifani P, Snounou G, Rénia L, Russell B (September 2017). "Strict tropism for CD71+/CD234+human reticulocytes limits the zoonotic potential ofPlasmodium cynomolgi". Blood. 130 (11): 1357–1363. doi:10.1182/blood-2017-02-764787. PMC 5600141. PMID 28698207.
- "Entrez Gene: Duffy antigen".
- Cutbush M, Mollison PL, Parkin DM (4 February 1950). "A New Human Blood Group". Nature. 165 (188–189): 188–189. Bibcode:1950Natur.165..188C. doi:10.1038/165188b0. S2CID 4265241.
- Tournamille C, Colin Y, Cartron JP, Le Van KC (Jun 1995). "Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals". Nat Genet. 10 (2): 224–8. doi:10.1038/ng0695-224. PMID 7663520. S2CID 7125832.
- Yazdanbakhsh K, Rios M, Storry JR, Kosower N, Parasol N, Chaudhuri A, Reid ME (March 2000). "Molecular mechanisms that lead to reduced expression of duffy antigens". Transfusion. 40 (3): 310–20. doi:10.1046/j.1537-2995.2000.40030310.x. PMID 10738032. S2CID 43747569.
- ^ Hamblin MT, Di Rienzo A (May 2000). "Detection of the signature of natural selection in humans: evidence from the Duffy blood group locus". American Journal of Human Genetics. 66 (5): 1669–79. doi:10.1086/302879. PMC 1378024. PMID 10762551.
- Hamblin MT, Thompson EE, Di Rienzo A (February 2002). "Complex Signatures of Natural Selection at the Duffy Blood Group Locus". American Journal of Human Genetics. 70 (2): 369–83. doi:10.1086/338628. PMC 419988. PMID 11753822.
- Zimmerman PA, Woolley I, Masinde GL, Miller SM, McNamara DT, Hazlett F, Mgone CS, Alpers MP, Genton B, Boatin BA, Kazura JW (November 1999). "Emergence of FY*Anull in a Plasmodium vivax-endemic region of Papua New Guinea". Proc. Natl. Acad. Sci. U.S.A. 96 (24): 13973–7. Bibcode:1999PNAS...9613973Z. doi:10.1073/pnas.96.24.13973. PMC 24175. PMID 10570183.
- Awasthi G, Dashb AP, Dasa A (September 2009). "Evolutionary insights into duffy gene in mammalian taxa with comparative genetic analysis". J Vector Borne Dis. 46 (3): 230–6. PMID 19724088.
- Tung J, Primus A, Bouley AJ, Severson TF, Alberts SC, Wray GA (July 2009). "Evolution of a malaria resistance gene in wild primates". Nature. 460 (7253): 388–91. Bibcode:2009Natur.460..388T. doi:10.1038/nature08149. PMID 19553936. S2CID 4430340.
- Schmid P, Ravenell KR, Sheldon SL, Flegel WA (2011). "DARC alleles and Duffy phenotypes in African Americans". Transfusion. 52 (6): 1260–1267. doi:10.1111/j.1537-2995.2011.03431.x. PMC 3374024. PMID 22082243.
- Oliveira TY, Harris EE, Meyer D, Jue CK, Silva WA (2012). "Molecular evolution of a malaria resistance gene (DARC) in primates". Immunogenetics. 64 (7): 497–505. doi:10.1007/s00251-012-0608-2. PMID 22395823. S2CID 17624523.
- Grodecka M, Bertrand O, Karolak E, Lisowski M, Waśniowska K (2012). "One-step immunopurification and lectinochemical characterization of the Duffy atypical chemokine receptor from human erythrocytes". Glycoconj. J. 29 (2–3): 93–105. doi:10.1007/s10719-011-9367-9. PMC 3311851. PMID 22246380.
- ^ Woolley IJ, Hotmire KA, Sramkoski RM, Zimmerman PA, Kazura JW (August 2000). "Differential expression of the duffy antigen receptor for chemokines according to RBC age and FY genotype". Transfusion. 40 (8): 949–53. doi:10.1046/j.1537-2995.2000.40080949.x. PMID 10960522. S2CID 46112984.
- Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC (1997). "Expression of chemokine receptors by subsets of neurons in the central nervous system". Journal of Immunology. 158 (6): 2882–90. doi:10.4049/jimmunol.158.6.2882. PMID 9058825. S2CID 12262317.
- Hadley TJ, Lu ZH, Wasniowska K, Martin AW, Peiper SC, Hesselgesser J, Horuk R (1994). "Postcapillary venule endothelial cells in kidney express a multispecific chemokine receptor that is structurally and functionally identical to the erythroid isoform, which is the Duffy blood group antigen". J. Clin. Invest. 94 (3): 985–91. doi:10.1172/JCI117465. PMC 295143. PMID 8083383.
- Rot A, Gutjahr JC, Biswas A, Aslani M, Hub E, Thiriot A, von Andrian UH, Megens RT, Weber C, Duchene J (2022). "Murine bone marrow macrophages and human monocytes do not express atypical chemokine receptor 1". Cell Stem Cell. 29 (7): 1013–1015. doi:10.1016/j.stem.2021.11.010. PMID 35803222. S2CID 250366123.
- Peiper SC, Wang ZX, Neote K, Martin AW, Showell HJ, Conklyn MJ, Ogborne K, Hadley TJ, Lu ZH, Hesselgesser J, Horuk R (April 1995). "The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor". J. Exp. Med. 181 (4): 1311–7. doi:10.1084/jem.181.4.1311. PMC 2191961. PMID 7699323.
- Chaudhuri A, Zbrzezna V, Polyakova J, Pogo AO, Hesselgesser J, Horuk R (1994). "Expression of the Duffy antigen in K562 cells. Evidence that it is the human erythrocyte chemokine receptor". J. Biol. Chem. 269 (11): 7835–8. doi:10.1016/S0021-9258(17)37123-5. PMID 8132497.
- Horuk R, Wang ZX, Peiper SC, Hesselgesser J (1994). "Identification and characterization of a promiscuous chemokine-binding protein in a human erythroleukemic cell line". J. Biol. Chem. 269 (26): 17730–3. doi:10.1016/S0021-9258(17)32501-2. PMID 7517400.
- Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, Miller LH (August 1993). "A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor". Science. 261 (5125): 1182–4. Bibcode:1993Sci...261.1182H. doi:10.1126/science.7689250. PMID 7689250.
- McMorran BJ, Wieczorski L, Drysdale KE, Chan JA, Huang HM, Smith C, Mitiku C, Beeson JG, Burgio G, Foote SJ (2012). "Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum". Science. 338 (6112): 1348–51. Bibcode:2012Sci...338.1348M. doi:10.1126/science.1228892. PMID 23224555. S2CID 206544569.
- Lu ZH, Wang ZX, Horuk R, Hesselgesser J, Lou YC, Hadley TJ, Peiper SC (1995). "The promiscuous chemokine binding profile of the Duffy antigen/receptor for chemokines is primarily localized to sequences in the amino-terminal domain". J. Biol. Chem. 270 (44): 26239–45. doi:10.1074/jbc.270.44.26239. PMID 7592830.
- Chaudhuri A, Zbrzezna V, Polyakova J, Pogo AO, Hesselgesser J, Horuk R (March 1994). "Expression of the Duffy antigen in K562 cells. Evidence that it is the human erythrocyte chemokine receptor". J. Biol. Chem. 269 (11): 7835–8. doi:10.1016/S0021-9258(17)37123-5. PMID 8132497.
- Fukuma N, Akimitsu N, Hamamoto H, Kusuhara H, Sugiyama Y, Sekimizu K (2003). "A role of the Duffy antigen for the maintenance of plasma chemokine concentrations". Biochem. Biophys. Res. Commun. 303 (1): 137–9. doi:10.1016/S0006-291X(03)00293-6. PMID 12646177.
- Lee JS, Frevert CW, Wurfel MM, Peiper SC, Wong VA, Ballman KK, Ruzinski JT, Rhim JS, Martin TR, Goodman RB (2003). "Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo". Journal of Immunology. 170 (10): 5244–51. doi:10.4049/jimmunol.170.10.5244. PMC 4357319. PMID 12734373.
- Dawson TC, Lentsch AB, Wang Z, Cowhig JE, Rot A, Maeda N, Peiper SC (2000). "Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC)". Blood. 96 (5): 1681–4. doi:10.1182/blood.V96.5.1681. PMID 10961863.
- Patterson AM, Siddall H, Chamberlain G, Gardner L, Middleton J (2002). "Expression of the duffy antigen/receptor for chemokines (DARC) by the inflamed synovial endothelium". J. Pathol. 197 (1): 108–16. doi:10.1002/path.1100. PMID 12081195. S2CID 2948161.
- Liu XH, Hadley TJ, Xu L, Peiper SC, Ray PE (1999). "Up-regulation of Duffy antigen receptor expression in children with renal disease". Kidney Int. 55 (4): 1491–500. doi:10.1046/j.1523-1755.1999.00385.x. PMID 10201015.
- Rot A (2005). "Contribution of Duffy antigen to chemokine function". Cytokine Growth Factor Rev. 16 (6): 687–94. doi:10.1016/j.cytogfr.2005.05.011. PMID 16054417.
- Segerer S, Regele H, MacK M, Kain R, Cartron JP, Colin Y, Kerjaschki D, Schlöndorff D (2000). "The Duffy antigen receptor for chemokines is up-regulated during acute renal transplant rejection and crescentic glomerulonephritis". Kidney Int. 58 (4): 1546–56. doi:10.1046/j.1523-1755.2000.00316.x. PMID 11012889.
- Segerer S, Cui Y, Eitner F, Goodpaster T, Hudkins KL, Mack M, Cartron JP, Colin Y, Schlondorff D, Alpers CE (2001). "Expression of chemokines and chemokine receptors during human renal transplant rejection". Am. J. Kidney Dis. 37 (3): 518–31. CiteSeerX 10.1.1.408.1801. doi:10.1016/S0272-6386(01)80009-3. PMID 11228176.
- Brühl H, Vielhauer V, Weiss M, Mack M, Schlöndorff D, Segerer S (2005). "Expression of DARC, CXCR3 and CCR5 in giant cell arteritis". Rheumatology. 44 (3): 309–13. doi:10.1093/rheumatology/keh485. PMID 15572394.
- Middleton J, Americh L, Gayon R, Julien D, Mansat M, Mansat P, Anract P, Cantagrel A, Cattan P, Reimund JM, Aguilar L, Amalric F, Girard JP (2005). "A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146". J. Pathol. 206 (3): 260–8. doi:10.1002/path.1788. PMID 15887283. S2CID 19284627.
- Segerer S, Böhmig GA, Exner M, Colin Y, Cartron JP, Kerjaschki D, Schlöndorff D, Regele H (2003). "When renal allografts turn DARC". Transplantation. 75 (7): 1030–4. doi:10.1097/01.TP.0000054679.91112.6F. PMID 12698093. S2CID 22739165.
- Pruenster M, Rot A (2006). "Throwing light on DARC". Biochem. Soc. Trans. 34 (Pt 6): 1005–8. doi:10.1042/BST0341005. PMID 17073738.
- Chakera A, Seeber RM, John AE, Eidne KA, Greaves DR (May 2008). "The duffy antigen/receptor for chemokines exists in an oligomeric form in living cells and functionally antagonizes CCR5 signaling through hetero-oligomerization". Mol. Pharmacol. 73 (5): 1362–70. doi:10.1124/mol.107.040915. PMID 18230715. S2CID 11847880.
- Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, Richmond A, Graham GJ, Segerer S, Nibbs RJ, Rot A (January 2009). "The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity". Nat. Immunol. 10 (1): 101–8. doi:10.1038/ni.1675. PMC 3205989. PMID 19060902.
- Levinson W (2004). Medical microbiology & immunology: examination & board review. New York: Lange Medical Books/McGraw-Hill. ISBN 978-0-07-143199-6.
- ^ Nickel RG, Willadsen SA, Freidhoff LR, Huang SK, Caraballo L, Naidu RP, Levett P, Blumenthal M, Banks-Schlegel S, Bleecker E, Beaty T, Ober C, Barnes KC (August 1999). "Determination of Duffy genotypes in three populations of African descent using PCR and sequence-specific oligonucleotides". Hum. Immunol. 60 (8): 738–42. doi:10.1016/S0198-8859(99)00039-7. PMID 10439320.
- Miller LH, Mason SJ, Clyde DF, McGinniss MH (August 1976). "The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy". N. Engl. J. Med. 295 (6): 302–4. doi:10.1056/NEJM197608052950602. PMID 778616.
- Estalote AC, Proto-Siqueira R, Silva WA, Zago MA, Palatnik M (2005). "The mutation G298A→Ala100Thr on the coding sequence of the Duffy antigen/chemokine receptor gene in non-caucasian Brazilians". Genet. Mol. Res. 4 (2): 166–73. PMID 16110438.
- Bauduer F, Feingold J, Lacombe D (October 2005). "The Basques: review of population genetics and Mendelian disorders". Hum. Biol. 77 (5): 619–37. doi:10.1353/hub.2006.0001. PMID 16596943. S2CID 46134067.
- Ferri G, Bini C, Ceccardi S, Ingravallo F, Lugaresi F, Pelotti S (March 2006). "Minisequencing-based genotyping of Duffy and ABO blood groups for forensic purposes". J. Forensic Sci. 51 (2): 357–60. doi:10.1111/j.1556-4029.2006.00058.x. PMID 16566771. S2CID 7461139.
- Das MK, Singh SS, Adak T, Vasantha K, Mohanty D (June 2005). "The Duffy blood groups of Jarawas - the primitive and vanishing tribe of Andaman and Nicobar Islands of India". Transfus Med. 15 (3): 237–40. doi:10.1111/j.1365-3148.2005.00583.x. PMID 15943709. S2CID 19301986.
- Kobyliansky E, Micle S, Goldschmidt-Nathan M, Arensburg B, Nathan H (1980). "Duffy, Kell and P blood group systems in some Jewish populations of Israel". Acta Anthropogenet. 4 (3–4): 173–9. PMID 7346047.
- Parasol N, Cohen N, Zemishlany Z, Lerer B, Kosower NS (April 2001). "Duffy antigen/receptor for chemokines (DARC): genotypes in Ashkenazi and non-Ashkenazi Jews in Israel". Hum. Biol. 73 (2): 307–13. doi:10.1353/hub.2001.0024. PMID 11446431. S2CID 25812715.
- Yan L, Zhu F, Fu Q, He J (2020). "ABO, Rh, MNS, Duffy, Kidd, Yt, Scianna, and Colton blood group systems in indigenous Chinese". Immunohematology. 21 (1): 10–4. doi:10.21307/immunohematology-2019-386. PMID 15783300.
- Chiaroni J, Touinssi M, Frassati C, Degioanni A, Gibert M, Reviron D, Mercier P, Boëtsch G (August 2004). "Genetic characterization of the population of Grande Comore Island (Njazidja) according to major blood groups". Hum. Biol. 76 (4): 527–41. doi:10.1353/hub.2004.0053. PMID 15754970. S2CID 36391602.
- Thakral B, Saluja K, Sharma RR, Marwaha N (June 2010). "Phenotype frequencies of blood group systems (Rh, Kell, Kidd, Duffy, MNS, P, Lewis, and Lutheran) in north Indian blood donors". Transfus Apher Sci. 43 (1): 17–22. doi:10.1016/j.transci.2010.05.006. PMID 20558108.
- Rosenberg R (May 2007). "Plasmodium vivax in Africa: hidden in plain sight?". Trends in Parasitology. 23 (5): 193–6. doi:10.1016/j.pt.2007.02.009. PMID 17360237.
- Mathews HM, Armstrong JC (March 1981). "Duffy blood types and vivax malaria in Ethiopia". The American Journal of Tropical Medicine and Hygiene. 30 (2): 299–303. doi:10.4269/ajtmh.1981.30.299. PMID 7015888.
- Sellami MH, Kaabi H, Midouni B, Dridi A, Mojaat N, Boukef MK, Hmida S (2008). "Duffy blood group system genotyping in an urban Tunisian population". Annals of Human Biology. 35 (4): 406–15. doi:10.1080/03014460802082127. PMID 18608113. S2CID 27486979.
- Lepers JP, Simonneau M, Charmot G (1986). "". Bull Soc Pathol Exot Filiales (in French). 79 (3): 417–20. PMID 3769126.
- Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, Zimmerman PA, Barnadas C, Beall CM, Gebremedhin A, Ménard D, Williams TN, Weatherall DJ, Hay SI (April 2011). "The global distribution of the Duffy blood group". Nat Commun. 2 (4): 266. Bibcode:2011NatCo...2..266H. doi:10.1038/ncomms1265. PMC 3074097. PMID 21468018.
- Keramati MR, Shakibaei H, Kheiyyami MI, Ayatollahi H, Badiei Z, Samavati M, Sadeghian MH (October 2011). "Blood group antigens frequencies in the northeast of Iran". Transfus. Apher. Sci. 45 (2): 133–6. doi:10.1016/j.transci.2011.07.006. PMID 21840761.
- Chittoria A, Mohanty S, Jaiswal YK, Das A (2012). "Natural Selection Mediated Association of the Duffy (FY) Gene Polymorphisms with Plasmodium vivax Malaria in India". PLOS ONE. 7 (9): e45219. Bibcode:2012PLoSO...745219C. doi:10.1371/journal.pone.0045219. PMC 3448599. PMID 23028857.
- ^ Ryan JR, Stoute JA, Amon J, Dunton RF, Mtalib R, Koros J, Owour B, Luckhart S, Wirtz RA, Barnwell JW, Rosenberg R (October 2006). "Evidence for transmission of Plasmodium vivax among a duffy antigen negative population in Western Kenya". Am. J. Trop. Med. Hyg. 75 (4): 575–81. doi:10.4269/ajtmh.2006.75.575. PMID 17038676.
- Ménard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, Thonier V, Carod JF, Domarle O, Colin Y, Bertrand O, Picot J, King CL, Grimberg BT, Mercereau-Puijalon O, Zimmerman PA (March 2010). "Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people". Proc. Natl. Acad. Sci. U.S.A. 107 (13): 5967–71. Bibcode:2010PNAS..107.5967M. doi:10.1073/pnas.0912496107. PMC 2851935. PMID 20231434.
- Mendes C, Dias F, Figueiredo J, Mora VG, Cano J, de Sousa B, do Rosário VE, Benito A, Berzosa P, Arez AP (June 2011). Franco-Paredes C (ed.). "Duffy Negative Antigen Is No Longer a Barrier to Plasmodium vivax – Molecular Evidences from the African West Coast (Angola and Equatorial Guinea)". PLOS Negl Trop Dis. 5 (6): e1192. doi:10.1371/journal.pntd.0001192. PMC 3119644. PMID 21713024.
- Wurtz N, Mint Lekweiry K, Bogreau H, Pradines B, Rogier C, Ould Mohamed Salem Boukhary A, Hafid JE, Ould Ahmedou Salem MS, Trape JF, Basco LK, Briolant S (2011). "Vivax malaria in Mauritania includes infection of a Duffy-negative individual". Malar. J. 10: 336. doi:10.1186/1475-2875-10-336. PMC 3228859. PMID 22050867.
- Cavasini CE, de Mattos LC, Couto AA, Couto VS, Gollino Y, Moretti LJ, Bonini-Domingos CR, Rossit AR, Castilho L, Machado RL (2007). "Duffy blood group gene polymorphisms among malaria vivax patients in four areas of the Brazilian Amazon region". Malar. J. 6: 167. doi:10.1186/1475-2875-6-167. PMC 2244634. PMID 18093292.
- Cavasini CE, Mattos LC, Couto AA, Bonini-Domingos CR, Valencia SH, Neiras WC, Alves RT, Rossit AR, Castilho L, Machado RL (October 2007). "Plasmodium vivax infection among Duffy antigen-negative individuals from the Brazilian Amazon region: an exception?". Trans. R. Soc. Trop. Med. Hyg. 101 (10): 1042–4. doi:10.1016/j.trstmh.2007.04.011. PMID 17604067.
- Culleton R, Ndounga M, Zeyrek FY, Coban C, Casimiro PN, Takeo S, Tsuboi T, Yadava A, Carter R, Tanabe K (November 2009). "Evidence for the transmission of Plasmodium vivax in the Republic of the Congo, West Central Africa". J. Infect. Dis. 200 (9): 1465–9. doi:10.1086/644510. PMID 19803728.
- Dhorda M, Nyehangane D, Rénia L, Piola P, Guerin PJ, Snounou G (2011). "Transmission of Plasmodium vivax in south-western Uganda: report of three cases in pregnant women". PLOS ONE. 6 (5): e19801. Bibcode:2011PLoSO...619801D. doi:10.1371/journal.pone.0019801. PMC 3094453. PMID 21603649.
- Carvalho TA, Queiroz MG, Cardoso GL, Diniz IG, Silva AN, Pinto AY, Guerreiro JF (December 2012). "Plasmodium vivax infection in Anajas, State of Para: no differential resistance profile among Duffy-negative and Duffy-positive individuals". Malar. J. 11 (1): 430. doi:10.1186/1475-2875-11-430. PMC 3544589. PMID 23259672.
- McHenry AM, Barnwell JW, Adams JH (October 2009). "Plasmodium vivax DBP binding to Aotus nancymaae erythrocytes is Duffy antigen dependent". J. Parasitol. 96 (1): 225–227. doi:10.1645/GE-2281.1. PMC 2883003. PMID 19799492.
- Albuquerque SR, Cavalcante Fde O, Sanguino EC, Tezza L, Chacon F, Castilho L, dos Santos MC (February 2010). "FY polymorphisms and vivax malaria in inhabitants of Amazonas State, Brazil". Parasitol Res. 106 (5): 1049–53. doi:10.1007/s00436-010-1745-x. PMID 20162434. S2CID 35436028.
- King CL, Adams JH, Xianli J, Grimberg BT, McHenry AM, Greenberg LJ, Siddiqui A, Howes RE, da Silva-Nunes M, Ferreira MU, Zimmerman PA (November 2011). "Fya/Fyb antigen polymorphism in human erythrocyte Duffy antigen affects susceptibility to Plasmodium vivax malaria". Proc Natl Acad Sci U S A. 108 (50): 20113–8. Bibcode:2011PNAS..10820113K. doi:10.1073/pnas.1109621108. PMC 3250126. PMID 22123959.
- McMorran BJ, Wieczorski L, Drysdale KE, Chan JA, Huang HM, Smith C, Mitiku C, Beeson JG, Burgio G, Foote SJ (December 2012). "Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum". Science. 338 (6112): 1348–51. Bibcode:2012Sci...338.1348M. doi:10.1126/science.1228892. PMID 23224555. S2CID 206544569.
- Sampath S, Carrico C, Janes J, Gurumoorthy S, Gibson C, Melcher M, Chitnis CE, Wang R, Schief WR, Smith JD (June 2013). "Glycan Masking of Plasmodium vivax Duffy Binding Protein for Probing Protein Binding Function and Vaccine Development". PLOS Pathog. 9 (6): e1003420. doi:10.1371/journal.ppat.1003420. PMC 3681752. PMID 23853575.
- Kano FS, de Souza AM, de Menezes Torres L, Costa MA, Souza-Silva FA, Sanchez BA, Fontes CJ, Soares IS, de Brito CF, Carvalho LH, Sousa TN (September 2018). "Susceptibility to Plasmodium vivax malaria associated with DARC (Duffy antigen) polymorphisms is influenced by the time of exposure to malaria". Scientific Reports. 8 (1): 13851. Bibcode:2018NatSR...813851K. doi:10.1038/s41598-018-32254-z. PMC 6138695. PMID 30218021.
- Vergara C, Tsai YJ, Grant AV, Rafaels N, Gao L, Hand T, Stockton M, Campbell M, Mercado D, Faruque M, Dunston G, Beaty TH, Oliveira RR, Ponte EV, Cruz AA, Carvalho E, Araujo MI, Watson H, Schleimer RP, Caraballo L, Nickel RG, Mathias RA, Barnes KC (November 2008). "Gene Encoding Duffy Antigen/Receptor for Chemokines Is Associated with Asthma and IgE in Three Populations". Am. J. Respir. Crit. Care Med. 178 (10): 1017–22. doi:10.1164/rccm.200801-182OC. PMC 2582596. PMID 18827265.
- ^ Duchene J, Novitzky-Basso I, Thiriot A, Casanova-Acebes M, Bianchini M, Etheridge SL, Hub E, Nitz K, Artinger K, Eller K, Caamaño J, Rülicke T, Moss P, Megens RT, von Andrian UH, Hidalgo A, Weber C, Rot A (May 2017). "Atypical chemokine receptor 1 on nucleated erythroid cells regulates hematopoiesis". Nature Immunology. 18 (7): 753–761. doi:10.1038/ni.3763. PMC 5480598. PMID 28553950.
- ^ Rigby A. "How Ancestry Shapes Our Immune Cells".
- Merz LE, Story CM, Osei MA, Jolley K, Ren S, Park HS, et al. (February 2023). "Absolute neutrophil count by Duffy status among healthy Black and African American adults". Blood Advances. 7 (3): 317–320. doi:10.1182/bloodadvances.2022007679. PMC 9881043. PMID 35994632.
- Legge SE, Christensen RH, Petersen L, Pardiñas AF, Bracher-Smith M, Knapper S, et al. (December 2020). "The Duffy-null genotype and risk of infection". Human Molecular Genetics. 29 (20): 3341–3349. doi:10.1093/hmg/ddaa208. PMC 7906776. PMID 32959868.
- Reich D, Nalls MA, Kao WH, Akylbekova EL, Tandon A, Patterson N, et al. (January 2009). Visscher PM (ed.). "Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene". PLOS Genetics. 5 (1): e1000360. doi:10.1371/journal.pgen.1000360. PMC 2628742. PMID 19180233.
- Merz LE, Story CM, Osei MA, Jolley K, Ren S, Park HS, et al. (February 2023). "Absolute neutrophil count by Duffy status among healthy Black and African American adults". Blood Advances. 7 (3): 317–320. doi:10.1182/bloodadvances.2022007679. PMC 9881043. PMID 35994632.
- Legge SE, Pardiñas AF, Helthuis M, Jansen JA, Jollie K, Knapper S, et al. (March 2019). "A genome-wide association study in individuals of African ancestry reveals the importance of the Duffy-null genotype in the assessment of clozapine-related neutropenia" (PDF). Molecular Psychiatry. 24 (3): 328–337. doi:10.1038/s41380-018-0335-7. PMID 30647433. S2CID 58010066.
- Naidoo KK, Ngubane A, Gaza P, Moodley A, Ndung'u T, Thobakgale CF (2019). "Neutrophil Effector Functions Are Not Impaired in Duffy Antigen Receptor for Chemokines (DARC)-Null Black South Africans". Frontiers in Immunology. 10: 551. doi:10.3389/fimmu.2019.00551. PMC 6443851. PMID 30972057.
- Zijlstra A, Quigley JP (September 2006). "The DARC side of metastasis: shining a light on KAI1-mediated metastasis suppression in the vascular tunnel". Cancer Cell. 10 (3): 177–8. doi:10.1016/j.ccr.2006.08.012. PMID 16959609.
- Wang J, Ou ZL, Hou YF, Luo JM, Shen ZZ, Ding J, Shao ZM (November 2006). "Enhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential". Oncogene. 25 (54): 7201–11. doi:10.1038/sj.onc.1209703. PMID 16785997.
- Mayr FB, Spiel AO, Leitner JM, Firbas C, Jilma-Stohlawetz P, Chang JY, Key NS, Jilma B (April 2009). "Racial differences in endotoxin-induced tissue factor-triggered coagulation". J. Thromb. Haemost. 7 (4): 634–40. doi:10.1111/j.1538-7836.2009.03307.x. PMID 19187081. S2CID 23613539.
- ^ He W, Neil S, Kulkarni H, Wright E, Agan BK, Marconi VC, Dolan MJ, Weiss RA, Ahuja SK (July 2008). "Duffy Antigen Receptor for Chemokines Mediates trans-Infection of HIV-1 from Red Blood Cells to Target Cells and Affects HIV-AIDS Susceptibility". Cell Host Microbe. 4 (1): 52–62. doi:10.1016/j.chom.2008.06.002. PMC 2562426. PMID 18621010.
- Kulkarni H, Marconi VC, He W, Landrum ML, Okulicz JF, Delmar J, Kazandjian D, Castiblanco J, Ahuja SS, Wright EJ, Weiss RA, Clark RA, Dolan MJ, Ahuja SK (July 2009). "The Duffy-null state is associated with a survival advantage in leukopenic HIV-infected persons of African ancestry". Blood. 114 (13): 2783–92. doi:10.1182/blood-2009-04-215186. PMC 2927046. PMID 19620399.
- Ramsuran V, Kulkarni H, He W, Mlisana K, Wright EJ, Werner L, Castiblanco J, Dhanda R, Le T, Dolan MJ, Guan W, Weiss RA, Clark RA, Karim SS, Ahuja SK, Ndung'u T (May 2011). "Duffy-Null–Associated Low Neutrophil Counts Influence HIV-1 Susceptibility in High-Risk South African Black Women". Clin. Infect. Dis. 52 (10): 1248–56. doi:10.1093/cid/cir119. PMC 3115278. PMID 21507922.
- Voruganti VS, Laston S, Haack K, Mehta NR, Smith CW, Cole SA, Butte NF, Comuzzie AG (September 2012). "Genome-wide association replicates the association of Duffy antigen receptor for chemokines (DARC) polymorphisms with serum monocyte chemoattractant protein-1 (MCP-1) levels in Hispanic children". Cytokine. 60 (3): 634–8. doi:10.1016/j.cyto.2012.08.029. PMC 3501981. PMID 23017229.
- Sidore C, Busonero F, Maschio A, Porcu E, Naitza S, Zoledziewska M, et al. (2015). "Genome sequencing elucidates Sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers". Nature Genetics. 47 (11): 1272–81. doi:10.1038/ng.3368. PMC 4627508. PMID 26366554.
- Smith E, McGettrick HM, Stone MA, Shaw JS, Middleton J, Nash GB, Buckley CD, Ed Rainger G (July 2008). "Duffy antigen receptor for chemokines and CXCL5 are essential for the recruitment of neutrophils in a multicellular model of rheumatoid arthritis synovium". Arthritis and Rheumatism. 58 (7): 1968–73. doi:10.1002/art.23545. PMC 2821686. PMID 18576313.
- Geleff S, Draganovici D, Jaksch P, Segerer S (July 2009). "The role of chemokine receptors in acute lung allograft rejection". Eur. Respir. J. 35 (1): 167–75. doi:10.1183/09031936.00042309. PMID 19608592.
- Guler N, Turgut M, Ozatli D, Turgut Y, Gokce AK, Koc S, Albayrak D (March 2009). "High ratio of Duffy (a+b+) phenotype in patients with multiple myeloma compared to healthy controls". Hematol Oncol. 27 (1): 50–1. doi:10.1002/hon.887. PMID 19206111. S2CID 20469787.
- Lee JS, Frevert CW, Thorning DR, Segerer S, Alpers CE, Cartron JP, Colin Y, Wong VA, Martin TR, Goodman RB (February 2003). "Enhanced expression of Duffy antigen in the lungs during suppurative pneumonia". J. Histochem. Cytochem. 51 (2): 159–66. doi:10.1177/002215540305100204. PMID 12533524.
- Elson JK, Beebe-Dimmer JL, Morgenstern H, Chilkuri M, Blanchard J, Lentsch AB (July 2010). "The Duffy Antigen/Receptor for Chemokines (DARC) and Prostate-Cancer Risk among Jamaican Men". J Immigr Minor Health. 13 (1): 36–41. doi:10.1007/s10903-010-9330-z. PMC 3017736. PMID 20596779.
- Watorek E, Boratyńska M, Hałoń A, Klinger M (2008). "Anti-Fya antibodies as the cause of an unfortunate post-transplant course in renal transplant recipient". Ann. Transplant. 13 (1): 48–52. PMID 18344944.
- Afenyi-Annan A, Kail M, Combs MR, Orringer EP, Ashley-Koch A, Telen MJ (May 2008). "Lack of Duffy antigen expression is associated with organ damage in patients with sickle cell disease". Transfusion. 48 (5): 917–24. doi:10.1111/j.1537-2995.2007.01622.x. PMID 18248572. S2CID 40367918.
- Nebor D, Durpes MC, Mougenel D, Mukisi-Mukaza M, Elion J, Hardy-Dessources MD, Romana M (July 2010). "Association between Duffy antigen receptor for chemokines expression and levels of inflammation markers in sickle cell anemia patients". Clin. Immunol. 136 (1): 116–22. doi:10.1016/j.clim.2010.02.023. PMID 20347396.
- Woolley IJ, Hutchinson P, Reeder JC, Kazura JW, Cortés A (2020). "Southeast Asian ovalocytosis is associated with increased expression of Duffy antigen receptor for chemokines (DARC)". Immunohematology. 25 (2): 63–6. doi:10.21307/immunohematology-2019-233. PMID 19927622.
- ^ Roback J, Combs MR, Grossman B, Hillyer C (2008). AABB Technical Manual (16th ed.). Bethesda: AABB Press.
- Klein HG, Anstee DJ (2005). Mollison's Blood Transfusion in Clinical Medicine (11th ed.). Oxford: Blackwell Publishing.
Further reading
- Pogo AO, Chaudhuri A (1996). "Duffy and receptors for P. vivax and chemotactic peptides". Transfusion Clinique et Biologique. 2 (4): 269–76. doi:10.1016/s1246-7820(05)80093-x. PMID 8542025.
- Raeymaekers P, Van Broeckhoven C, Backhovens H, Wehnert A, Muylle L, De Jonghe P, Gheuens J, Vandenberghe A (1988). "The Duffy blood group is linked to the alpha-spectrin locus in a large pedigree with autosomal dominant inheritance of Charcot-Marie-Tooth disease type 1". Hum. Genet. 78 (1): 76–8. doi:10.1007/BF00291239. PMID 2892777. S2CID 7905017.
- Tournamille C, Le Van Kim C, Gane P, Cartron JP, Colin Y (1995). "Molecular basis and PCR-DNA typing of the Fya/fyb blood group polymorphism". Hum. Genet. 95 (4): 407–10. doi:10.1007/BF00208965. PMID 7705836. S2CID 22363912.
- Iwamoto S, Omi T, Kajii E, Ikemoto S (1995). "Genomic organization of the glycoprotein D gene: Duffy blood group Fya/Fyb alloantigen system is associated with a polymorphism at the 44-amino acid residue". Blood. 85 (3): 622–6. doi:10.1182/blood.V85.3.622.bloodjournal853622. PMID 7833467.
- Tournamille C, Le Van Kim C, Gane P, Blanchard D, Proudfoot AE, Cartron JP, Colin Y (1997). "Close association of the first and fourth extracellular domains of the Duffy antigen/receptor for chemokines by a disulfide bond is required for ligand binding". J. Biol. Chem. 272 (26): 16274–80. doi:10.1074/jbc.272.26.16274. PMID 9195930.
- Tournamille C, Le Van Kim C, Gane P, Le Pennec PY, Roubinet F, Babinet J, Cartron JP, Colin Y (1998). "Arg89Cys substitution results in very low membrane expression of the Duffy antigen/receptor for chemokines in Fy(x) individuals". Blood. 92 (6): 2147–56. doi:10.1182/blood.V92.6.2147. PMID 9731074. S2CID 25725619.
- Parasol N, Reid M, Rios M, Castilho L, Harari I, Kosower NS (1998). "A novel mutation in the coding sequence of the FY*B allele of the Duffy chemokine receptor gene is associated with an altered erythrocyte phenotype". Blood. 92 (7): 2237–43. doi:10.1182/blood.V92.7.2237. PMID 9746760.
- Olsson ML, Smythe JS, Hansson C, Poole J, Mallinson G, Jones J, Avent ND, Daniels G (1999). "The Fy(x) phenotype is associated with a missense mutation in the Fy(b) allele predicting Arg89Cys in the Duffy glycoprotein". Br. J. Haematol. 103 (4): 1184–91. doi:10.1046/j.1365-2141.1998.01083.x. PMID 9886340. S2CID 7873621.
- Lachgar A, Jaureguiberry G, Le Buenac H, Bizzini B, Zagury JF, Rappaport J, Zagury D (1999). "Binding of HIV-1 to RBCs involves the Duffy antigen receptors for chemokines (DARC)". Biomed. Pharmacother. 52 (10): 436–9. doi:10.1016/S0753-3322(99)80021-3. PMID 9921412.
- Woolley IJ, Kalayjian R, Valdez H, Hamza N, Jacobs G, Lederman MM, Zimmerman PA (2002). "HIV nephropathy and the Duffy antigen/receptor for Chemokines in African Americans". J. Nephrol. 14 (5): 384–7. PMID 11730271.
External links
- DARC+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Duffy at BGMUT Blood Group Antigen Gene Mutation Database at NCBI, NIH
- Duffy gene
- Population data
- Overview of all the structural information available in the PDB for UniProt: Q16570 (Atypical chemokine receptor 1) at the PDBe-KB.
| Proteins: clusters of differentiation (see also list of human clusters of differentiation) | |
|---|---|
| 1–50 | |
| 51–100 | |
| 101–150 | |
| 151–200 | |
| 201–250 | |
| 251–300 | |
| 301–350 | |
| Blood transfusion and transfusion medicine | |
|---|---|
| Blood products | |
| General concepts | |
| Methods | |
| Tests | |
| Transfusion reactions and adverse effects | |
| Blood group systems | |



