| L-xylulose reductase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 L-Xylulose reductase tetramer, Human L-Xylulose reductase tetramer, Human | |||||||||
| Identifiers | |||||||||
| EC no. | 1.1.1.10 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
| dicarbonyl/L-xylulose reductase | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Symbol | DCXR | ||||||
| NCBI gene | 51181 | ||||||
| HGNC | 18985 | ||||||
| OMIM | 608347 | ||||||
| RefSeq | NM_016286 | ||||||
| UniProt | Q7Z4W1 | ||||||
| Other data | |||||||
| EC number | 1.1.1.10 | ||||||
| Locus | Chr. 17 q25.3 | ||||||
| |||||||
Dicarbonyl/L-xylulose reductase, also known as carbonyl reductase II, is an enzyme that in human is encoded by the DCXR gene located on chromosome 17.
Structure
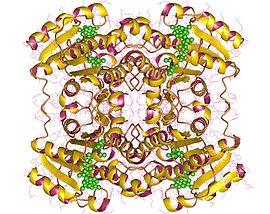
The DCXR gene encodes a membrane protein that is approximately 34 kDa in size and composed of 224 amino acids. The protein is highly expressed in the kidney and localizes to the cytoplasmic membrane.
Function
DCSR catalyzes the reduction of several L-xylylose as well as a number of pentoses, tetroses, trioses, alpha-dicarbonyl compounds. The enzyme is involved in carbohydrate metabolism, glucose metabolism, the uronate cycle and may play a role in the water absorption and cellular osmoregulation in the proximal renal tubules by producing xylitol.
In enzymology, an L-xylulose reductase (EC 1.1.1.10) is an enzyme that catalyzes the chemical reaction
- xylitol + NADP L-xylulose + NADPH + H
Thus, the two substrates of this enzyme are xylitol and NADP, whereas its 3 products are L-xylulose, NADPH, and H.
This enzyme belongs to the superfamily of short-chain oxidoreductases, specifically those acting on the CH-OH group of donor with NAD or NADP as acceptor. The systematic name of this enzyme class is xylitol:NADP 2-oxidoreductase (L-xylulose-forming).
Clinical significance
A deficiency is responsible for pentosuria. The insufficiency of L-xylulose reductase activity causes an inborn error of metabolism disease characterized by excessive urinary excretion of L-xylulose.
Over-expression and ectopic expression of the protein may be associated with prostate adenocarcinoma.
References
- Nakagawa J, Ishikura S, Asami J, Isaji T, Usami N, Hara A, Sakurai T, Tsuritani K, Oda K, Takahashi M, Yoshimoto M, Otsuka N, Kitamura K (2002). "Molecular characterization of mammalian dicarbonyl/L-xylulose reductase and its localization in kidney". J. Biol. Chem. 277 (20): 17883–91. doi:10.1074/jbc.M110703200. PMID 11882650.
- Zhao HT, Endo S, Ishikura S, Chung R, Hogg PJ, Hara A, El-Kabbani O (2009). "Structure/function analysis of a critical disulfide bond in the active site of L-xylulose reductase". Cell. Mol. Life Sci. 66 (9): 1570–9. doi:10.1007/s00018-009-9065-y. PMC 11131457. PMID 19337691. S2CID 8332906.
- Cho-Vega JH, Tsavachidis S, Do KA, Nakagawa J, Medeiros LJ, McDonnell TJ (2007). "Dicarbonyl/L-xylulose reductase: a potential biomarker identified by laser-capture microdissection-micro serial analysis of gene expression of human prostate adenocarcinoma". Cancer Epidemiol. Biomarkers Prev. 16 (12): 2615–22. doi:10.1158/1055-9965.EPI-07-0684. PMID 18086765.
External links
- L-xylulose+reductase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
| Enzymes | |
|---|---|
| Activity | |
| Regulation | |
| Classification | |
| Kinetics | |
| Types |
|
This EC 1.1.1 enzyme-related article is a stub. You can help Misplaced Pages by expanding it. |
 L-xylulose + NADPH + H
L-xylulose + NADPH + H