| |
| Names | |
|---|---|
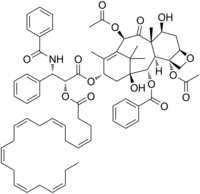
| IUPAC name 1,7β-Dihydroxy-9-oxo-5β,20-epoxytax-11-ene-2α,4α,10β,13α-tetrayl 4,10-diacetate 13-oxy}-3-phenylpropanoate] 2-benzoate | |
| Systematic IUPAC name (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-4,11-Dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanobenzoxetoannulene-6,9,12,12b-tetrayl 6,12b-diacetate 9-oxy}-3-phenylpropanoate] 12-benzoate | |
| Other names Docosahexaenoyl-paclitaxel; Taxoprexin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C69H81NO15 |
| Molar mass | 1164.399 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
DHA-paclitaxel (or Taxoprexin) is an investigational drug (from Protarga Inc) made by linking paclitaxel to docosahexaenoic acid (DHA), a fatty acid that is easily taken up by tumor cells; the DHA-paclitaxel “appears not to be cytotoxic until the bond with DHA is cleaved within the cell.” The advantage of DHA-paclitaxel over paclitaxel is DHA-paclitaxel's ability to carry much higher concentrations of paclitaxel to the cells, which are maintained for longer periods in the tumor cells, thus increasing their action. With increased activity, DHA-paclitaxel, also known as Taxoprexin, may have a more successful response in cancer patients than Taxol, and it may be able to treat more types of cancer than Taxol has been able to treat.
Clinical trials
In 2007, a phase II clinical trial reported "modest activity in patients with oesophago-gastric cancer".
References
- Whelan, Jo (2002). "Targeted taxane therapy for cancer". Drug Discovery Today. 7 (2): 90–92. doi:10.1016/S1359-6446(01)02149-3. PMID 11790612.
- Jones, RJ; Hawkins, RE; Eatock, MM; Ferry, DR; Eskens, FA; Wilke, H; Evans, TR (2008). "A phase II open-label study of DHA-paclitaxel (Taxoprexin) by 2-h intravenous infusion in previously untreated patients with locally advanced or metastatic gastric or oesophageal adenocarcinoma". Cancer Chemotherapy and Pharmacology. 61 (3): 435–41. doi:10.1007/s00280-007-0486-8. PMID 17440725.
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |