| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Alcohol by volume" – news · newspapers · books · scholar · JSTOR (October 2024) (Learn how and when to remove this message) |

Alcohol by volume (abbreviated as alc/vol or ABV) is a standard measure of the volume of alcohol contained in a given volume of an alcoholic beverage, expressed as a volume percent. It is defined as the number of millilitres (mL) of pure ethanol present in 100 mL (3.5 imp fl oz; 3.4 US fl oz) of solution at 20 °C (68 °F). The number of millilitres of pure ethanol is the mass of the ethanol divided by its density at 20 °C (68 °F), which is 0.78945 g/mL (0.82353 oz/US fl oz; 0.79122 oz/imp fl oz; 0.45633 oz/cu in). The alc/vol standard is used worldwide. The International Organization of Legal Metrology has tables of density of water–ethanol mixtures at different concentrations and temperatures.
In some countries, e.g. France, alcohol by volume is often referred to as degrees Gay-Lussac (after the French chemist Joseph Louis Gay-Lussac), although there is a slight difference since the Gay-Lussac convention uses the International Standard Atmosphere value for temperature, 15 °C (59 °F).
Volume change
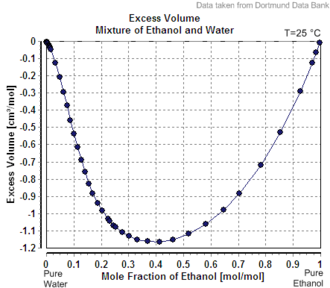
Mixing two solutions of alcohol of different strengths usually causes a change in volume. Mixing pure water with a solution less than 24% by mass causes a slight increase in total volume, whereas the mixing of two solutions above 24% causes a decrease in volume. The phenomenon of volume changes due to mixing dissimilar solutions is called "partial molar volume". Water and ethanol are both polar solvents. When water is added to ethanol, the smaller water molecules are attracted to the ethanol's hydroxyl group, and each molecule alters the polarity field of the other. The attraction allows closer spacing between molecules than is usually found in non-polar mixtures.
Thus, alc/vol is not the same as volume fraction expressed as a percentage. Volume fraction, which is widely used in chemistry (commonly denoted as v/v), is defined as the volume of a particular component divided by the sum of all components in the mixture when they are measured separately. For example, to make 100 mL of 50% alc/vol ethanol solution, water would be added to 50 mL of ethanol to make up exactly 100 mL. Whereas to make a 50% v/v ethanol solution, 50 mL of ethanol and 50 mL of water could be mixed but the resulting volume of solution will measure less than 100 mL due to the change of volume on mixing, and will contain a higher concentration of ethanol. The difference is not large, with the maximum difference being less than 2.5%, and less than 0.5% difference for concentrations under 20%.
Threshold levels
Legal thresholds
Some drinks have requirements of alcoholic content in order to be certified as a certain alcohol brand or label.
Low-alcohol beers (<0.5) are considered in some countries such as Iran as permitted (or "halal" under Muslim vocabulary) despite alcohol being banned. However, the level of alcohol-free beers is typically the lowest commercially sold 0.05.
Biological thresholds
It is near impossible for a healthy person to become intoxicated drinking low-alcohol drinks. The low concentration severely limits the rate of intake, which is easily dispatched by human metabolism. Quickly drinking 1.5 L of 0.4% alc/vol beer in an hour resulted in a maximum of 0.0056% BAC in a study of German volunteers. Healthy human kidneys can only excrete 0.8–1.0 L of water per hour, making water intoxication likely to set in before any alcoholic intoxication.
The process of ethanol fermentation will slow down and eventually come to a halt as the alcohol produced becomes too concentrated for the yeast to tolerate, defining an upper limit of alc/vol for non-distilled alcoholic drinks. The typical tolerance for beer yeasts is at 8–12%, while wine yeasts typically range from 14–18%, with speciality ones reaching 20% alc/vol. Any higher would require distillation, producing liquor.
Typical levels
Details about typical amounts of alcohol contained in various beverages can be found in the articles about them.
| Drink | Typical alc/vol | Lowest | Highest |
|---|---|---|---|
| Fruit juice (naturally occurring) | 0–0.11% They qualify as alcohol-free drinks in most countries.
(most juices do not have alcohol but orange or grape may have some from early fermentation) |
0.00 | 0.11 |
| Low-alcohol beer | 0.05–1.2% (usually not considered as alcohol legally)
Under 2.5% in Finland, and 2.25% in Sweden, however. |
0.05 | 1.02 |
| Kvass | 0.05–1.5% | 0.05 | 1.50 |
| Kefir | 0.2–2.0% | 0.20 | 2.00 |
| Sobia | 0.2–6.8% | 0.20 | 6.80 |
| Kombucha | 0.5–1.5% | 0.50 | 1.50 |
| Kumis | 0.7–4.5% (usually 0.7–2.5%) | 0.70 | 4.50 |
| Boza | 1.0% | 1.00 | 1.00 |
| Chicha | 1.0–11% (usually 1–6%) | 1.00 | 11.00 |
| Tubâ | 2.0–4.0% | 2.00 | 4.00 |
| Chūhai | 3.0–12.0% (usually 3–8%) | 3.00 | 12.00 |
| Pulque | 2.0–7.0% (usually 4–6%) | 2.00 | 12.00 |
| Beer | (usually 4–6%) | 2.00 | 10.00 |
| Cider | (usually 4–8%) | 4.00 | 8.00 |
| Palm wine | 4.0–6.0% | 4.00 | 6.00 |
| Alcopops | 4.0–17.5% | 4.00 | 17.50 |
| Malt liquor | 5.0-9.0% | 5.00 | 9.00 |
| Hard seltzer | 5.0% | 5.00 | 5.00 |
| Four Loko | 6–14% | 6.00 | 14.00 |
| Makgeolli | 6.5–7% | 6.50 | 7.00 |
| Kuchikamizake | 7% | 7.00 | 7.00 |
| Barley wine (strong ale) | 8–15% | 8.00 | 15.00 |
| Mead | 8–16% | 8.00 | 16.00 |
| Wine | 5.5–16% (most often 12.5–14.5%) | 5.50 | 16.00 |
| Bahalina | 10–13% | 10.50 | 13.00 |
| Basi | 10–16% | 10.00 | 16.00 |
| Bignay wine | 12–13% | 12.00 | 13.00 |
| Duhat wine | 12–13% | 12.00 | 13.00 |
| Tapuy | 14–19% | 14.00 | 19.00 |
| Kilju | 15–17% | 15.00 | 17.00 |
| Dessert wine | 14–25% | 14.00 | 25.00 |
| Sake | 15% (or 18–20% if not diluted prior to bottling) | 15.00 | 20.00 |
| Liqueurs | 15–55% | 15.50 | 55.00 |
| Fortified wine | 15.5–20% (in the European Union, 15–22%) | 15.50 | 22.00 |
| Soju | 14–45% (usually 17%) | 14.00 | 45.00 |
| Rice wine | 18–25% | 18.00 | 25.00 |
| Shochu | 25–45% (usually 25%) | 25.00 | 45.00 |
| Awamori | 25–60% (usually 30%) | 25.00 | 60.00 |
| Rượu đế | 27–45% (usually 35% – except Ruou tam – 40–45%) | 27.00 | 45.00 |
| Bitters | 28–45% | 28.00 | 45.00 |
| Applejack | 30–40% | 30.00 | 40.00 |
| Pisco | 30–48% | 30.00 | 48.00 |
| Țuică (Romanian drink) | 30–65% (usually 35–55%) | 30.00 | 65.00 |
| Mezcal, Tequila | 32–60% (usually 40%) | 32.00 | 60.00 |
| Vodka | 35–95% (usually 40%, minimum of 37.5% in the European Union) | 35.00 | 95.00 |
| Rum | 37.5–80% (usually 40%) | 37.50 | 80.00 |
| Brandy | 35–60% (usually 40%) | 35.00 | 60.00 |
| Grappa | 37.5–60% | 37.50 | 60.00 |
| Ouzo | 37.5% | 37.50 | 37.50 |
| Gin | 37.5–50% | 37.50 | 50.00 |
| Pálinka | 37.5–86% (usually 52%) | 37.50 | 86.00 |
| Cachaça | 38–48% | 38.00 | 48.00 |
| Sotol | 38–60% | 38.00 | 60.00 |
| Stroh | 38–80% | 38.00 | 80.00 |
| Fernet | 39–45% | 39.00 | 45.00 |
| Lambanog | 40–45% | 40.00 | 45.00 |
| Nalewka | 40–45% | 40.00 | 45.00 |
| Tsipouro | 40–45% | 40.00 | 45.00 |
| Rakı | 40–50% | 40.00 | 50.00 |
| Scotch whisky | 40–70+% | 40.00 | 70.00+ |
| Whisky | 40–70+% (usually 40%, 43% or 46%) | 40.00 | 70.00+ |
| Baijiu | 40–65% | 40.00 | 65.00 |
| Chacha | 40–70% | 40.00 | 70.00 |
| Bourbon whiskey | min 40% bottled | 40.00 | 80.00 |
| Rakija (Central/Southeast European drink) | 40–86% | 42.00 | 86.00 |
| Maotai | 43–53% | 43.00 | 53.00 |
| Absinthe | 45–89.9% | 45.00 | 89.90 |
| Arak | 60–65% | 60.00 | 65.00 |
| Oghi | 60–75% | 60.00 | 75.00 |
| Poitín | 60–95% | 60.00 | 95.00 |
| Centerbe (herb liqueur) | 70% | 70.00 | 70.00 |
| Neutral grain spirit | 85–95% | 85.00 | 95.00 |
| Cocoroco | 93–96% | 93.00 | 96.00 |
| Rectified spirit | 95% up to a practical limit of 97.2% | 95.00 | 97.20 |
Practical estimation of alcohol content
Main article: Gravity (alcoholic beverage)During the production of wine and beer, yeast is added to a sugary solution. During fermentation, the yeasts consume the sugars and produce alcohol. The density of sugar in water is greater than the density of alcohol in water. A hydrometer is used to measure the change in specific gravity (SG) of the solution before and after fermentation. The volume of alcohol in the solution can then be estimated. There are a number of empirical formulae which brewers and winemakers use to estimate the alcohol content of the liquor made.
Specific gravity is the density of a liquid relative to that of water, i.e., if the density of the liquid is 1.05 times that of water, it has a specific gravity of 1.05. In UK brewing usage, it is customary to regard the reference value for water to be 1000, so the specific gravity of the same example beer would be quoted as 1050. The formulas here assume that the former definition is used for specific gravity.
General
During ethanol fermentation the yeast converts one mole of sugar into two moles of alcohol. A general formula for calculating the resulting alcohol concentration by volume can be written:
where SBV fermented is sugar by volume (g/dL) converted to alcohol during fermentation and GECF is the glucose-ethanol conversion factor:
where 46.069 is the molar mass of ethanol and 180.156 is the molar mass of glucose and fructose.
Sugar by volume can be calculated from Brix (sugar by weight) and SG (relative density):
SG can be measured using an hydrometer and Brix can be calculated from SG. A simple formula for calculating Brix from SG is (SG 1.000 - 1.179):
By substituting Brix in the SBV formula above, we get a formula for calculating SBV from SG only:
By further substitution, we get a formula for calculating ABV from SG only:
The factor 135 is most accurate in the center of the SG drop range of 0.000 to 0.179. Since the correlation of SG and Brix is non-linear it is common to divide the range to increase accuracy when using the simple ABV formula:
| SG drop | ABV | Factor |
|---|---|---|
| 0.000 - 0.038 | 0 - 5 | 133 |
| 0.038 - 0.075 | 5 - 10 | 134 |
| 0.075 - 0.112 | 10 - 15 | 135 |
| 0.112 - 0.148 | 15 - 20 | 136 |
| 0.148 - 0.179 | 20 - 24 | 137 |
Advanced
Advanced formula derived from Carl Balling empirical formulas. The formula compensates for changes in SG with changes in alcohol concentration and for the fact that not all sugar is converted into alcohol. All values are measured at 20 degree C.
where SG final is the specific gravity when fermentation ends, Plato start is the sugar by weight when fermentation begins, Plato final is the sugar by weight when fermentation ends. Brix can be used insted of Plato as they are nearly identical.
Wine
The simplest method for wine has been described by English author Cyril Berry:
Beer
See also: Beer measurement § StrengthOne calculation for beer is:
For higher ABV above 6% many brewers use this formula:
Other methods of specifying alcohol content
Alcohol proof
Main article: Alcohol proofAnother way of specifying the amount of alcohol content is alcohol proof, which in the United States is twice the alcohol-by-volume (alc/vol) number. This may lead to confusion over similar products bought in varying regions that have different names on country-specific labels. For example, Stroh rum that is 80% ABV is advertised and labeled as Stroh 80 when sold in Europe, but is named Stroh 160 when sold in the United States.
In the United Kingdom, proof is 1.75 times the number (expressed as a percentage). For example, 40% alc/vol is 80 proof in the US and 70 proof in the UK. However, since 1980, alcohol proof in the UK has been replaced by alc/vol as a measure of alcohol content, avoiding confusion between the UK and US proof standards.
Alcohol by weight
In the United States, Arkansas, Kansas, Mississippi, South Carolina, and Tennessee regulate and tax alcoholic beverages according to alcohol by weight (ABW), expressed as a percentage of total mass. The alc/vol value of a beverage is always higher than the ABW.
Because ABW measures the proportion of the drink's mass which is alcohol, while alc/vol is the proportion of the drink's volume which is alcohol, the two values are in the same proportion as the drink's density is with the density of alcohol. Therefore, one can use the following equation to convert between ABV and ABW:
At relatively low alc/vol, the alcohol percentage by weight is about 4/5 of the alc/vol (e.g., 3.2% ABW is about 4% alc/vol). However, because of the miscibility of alcohol and water, the conversion factor is not constant but rather depends upon the concentration of alcohol.
See also
Portals:Notes
- See data in the CRC Handbook of Chemistry and Physics, 49th edition, pp. D-151 and D-152. Mixing a solution above 24% with a solution below 24% may cause an increase or a decrease, depending on the details.
References
- "Beer 101". Lafayette Brewing Co. Archived from the original on 19 February 2012. Retrieved 4 February 2012.
- "Glossary of whisky and distillation". celtic-whisky.com. Archived from the original on 12 February 2012. Retrieved 4 February 2012.
- "British Brewing Glossary". English Ales Brewery Monterey. Archived from the original on 19 February 2012. Retrieved 4 February 2012.
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 3.246. ISBN 978-1-4398-5511-9.
- "Joseph Louis Gay-Lussac (1778–1850)". chemistry.about.com. Archived from the original on 6 March 2008. Retrieved 5 July 2008.
- "Density ρ of Ethanol-Water Mixtures at the Temperature in °C Indicated by Superscript". CRC Handbook of Chemistry and Physics. Retrieved 13 December 2019.
This source gives density data for ethanol:water mixes by %weight ethanol in 5% increments and against temperature including at 25 °C, used here. It can be calculated from this table that at 25 °C, 45 g of ethanol has volume 57.3 mL, 55 g of water has volume 55.2 mL; these sum to 112.5 mL. When mixed they have volume 108.6 mL. - Hajebi, Amirali; Nasserinejad, Maryam; Rezaei, Negar; Azadnajafabad, Sina; Rashidi, Mohammad-Mahdi; Ahmadi, Naser; Ghasemi, Erfan; Farzi, Yosef; Yoosefi, Moein; Djalalinia, Shirin; Fattahi, Nima; Rezaei, Shahabeddin; Foroutan Mehr, Elmira; Kazemi, Ameneh; Haghshenas, Rosa (22 July 2024). "Alcohol consumption among Iranian population based on the findings of STEPS survey 2021". Scientific Reports. 14 (1): 16819. doi:10.1038/s41598-024-66257-w. ISSN 2045-2322. PMC 11263364.
- Thierauf, A.; Große Perdekamp, M.; Auwärter, V. (August 2012). "Maximale Blutalkoholkonzentration nach forciertem Konsum von alkoholfreiem Bier". Rechtsmedizin. 22 (4): 244–247. doi:10.1007/s00194-012-0835-8. S2CID 29586117.
- Ballantyne, Coco (21 June 2007). "Strange but True: Drinking Too Much Water Can Kill". Scientific American. Retrieved 31 August 2015.
- "Yeast Strains Chart". WineMakerMag.com. Retrieved 29 November 2023.
- Smith, Brad (19 December 2018). "Alcohol Tolerance in Beer Yeast and BeerSmith 3". BeerSmith. Retrieved 29 November 2023.
- Gorgus, Eva; Hittinger, Maike; Schrenk, Dieter (2016). "Estimates of Ethanol Exposure in Children from Food not Labeled as Alcohol-Containing". J Anal Toxicol. 40 (7). Oxford Academic: 537–42. doi:10.1093/jat/bkw046. PMC 5421578. PMID 27405361.
- "Brewing (and Chewing) the Origins of Sake". Boston Sake. 2 April 2012.
- Robinson 2006, p. 10.
- "Wine: From the Lightest to the Strongest". Wine Folly. 23 November 2015. Retrieved 20 June 2019.
- Robinson 2006, p. 279.
- Council Regulation (EC) No 479/2008; Annex IV, §3 (European Union document). "Liqueur wine", p. 46.
- "Caimán Alcohol Drink - Alcoholic Beverages". Package Inspiration. 10 January 2015. Retrieved 15 October 2024.
- Hall, Michael L. "Brew by the numbers, page 56-57" (PDF).
- ^ Berry 1998.
- "Get to Know Your Alcohol (By Volume)". BeerAdvocate.com. 18 June 2003. Archived from the original on 3 July 2014.
- Peros, Roko (7 May 2010). "Calculate Percent Alcohol in Beer". BrewMoreBeer.com.
- Regan 2003.
- Alexander, Amie (27 April 2024). "Definition of "Beer" in terms of ABV or ABW, by State" (PDF). Agricultural & Food Law Consortium. Archived (PDF) from the original on 27 April 2024. Retrieved 27 April 2024.
- "Alcohol Content In Beer". realbeer.com. Archived from the original on 4 July 2008. Retrieved 5 July 2008.
Bibliography
- Hehner, Otto (1880). Alcohol Tables: giving for all specific gravities, from 1.0000 to 0.7938, the percentages of absolute alcohol, by weight and volume. London: J & A Churchill. ASIN B0008B5HOU.
- Berry, C. J. J. (1998). First Steps in Winemaking. Nexus Special Interests. ISBN 978-1-85486-139-9.
- Regan, Gary (2003). The Joy of Mixology. Clarkson Potter. ISBN 978-0-609-60884-5.
- Robinson, Jancis (2006). The Oxford Companion to Wine (3rd ed.). Oxford: OUP. ISBN 978-0-19-860990-2.
External links
- "How do brewers measure the alcohol in beer?". HowStuffWorks. 12 December 2000.
- Jayes, Wayne. "Alcohol Strength and Density". sugartech.co.za. The Sugar Engineers.















