 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nipent |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692004 |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | 4% |
| Metabolism | Hepatic, minor |
| Elimination half-life | 2.6 to 16 hours, mean 5.7 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.991 |
| Chemical and physical data | |
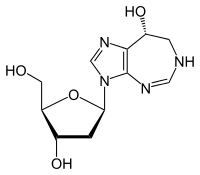
| Formula | C11H16N4O4 |
| Molar mass | 268.273 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Pentostatin (or deoxycoformycin, trade name Nipent, manufactured by SuperGen) is an anticancer chemotherapeutic drug.
Mechanism
It is classified as a purine analog, which is a type of antimetabolite.
It mimics the nucleoside adenosine and thus inhibits the enzyme adenosine deaminase, interfering with the cell's ability to process DNA.
Cancer cells generally divide more often than healthy cells; DNA is highly involved in cell division (mitosis) and drugs which target DNA-related processes are therefore more toxic to cancer cells than healthy cells.
Uses
Pentostatin is used to treat hairy cell leukemia. It is given by intravenous infusion once every two weeks for three to six months.
Additionally, pentostatin has been used to treat steroid-refractory acute and chronic graft-versus-host disease.
Pentostatin is also used in chronic lymphocytic leukemia (CLL) patients who have relapsed.
Synthesis
This is the original synthetic pathway although improvements were made.

References
- "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- Kay NE, Geyer SM, Call TG, et al. (January 2007). "Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia". Blood. 109 (2): 405–11. doi:10.1182/blood-2006-07-033274. PMC 1785105. PMID 17008537.
- Sauter C, Lamanna N, Weiss MA (September 2008). "Pentostatin in chronic lymphocytic leukemia". Expert Opin Drug Metab Toxicol. 4 (9): 1217–22. doi:10.1517/17425255.4.9.1217. PMID 18721115.
- Cannon T, Mobarek D, Wegge J, Tabbara IA (October 2008). "Hairy cell leukemia: current concepts". Cancer Invest. 26 (8): 860–5. doi:10.1080/07357900801965034. PMID 18798068.
- Bolaños-Meade J, Jacobsohn DA, Margolis J, Ogden A, Wientjes MG, Byrd JC, Lucas DM, Anders V, Phelps M, Grever MR, Vogelsang GB (April 2005). "Pentostatin in steroid-refractory acute graft-versus-host disease". J Clin Oncol. 23 (12): 2661–8. doi:10.1200/JCO.2005.06.130. PMID 15837980.
- Baker, D. C., Putt, S. R. (September 1979). "A total synthesis of pentostatin, the potent inhibitor of adenosine deaminase". Journal of the American Chemical Society. 101 (20): 6127–6128. doi:10.1021/ja00514a048.
- Showalter, H.D.Hollis; Putt, Sterling R (1981). "Studies related to the total synthesis of pentostatin: An efficient, regiospecific glycosylation of 6,7-dihydroimidazodiazepin-8(3H)-one and related homologs.". Tetrahedron Letters. 22 (33): 3155–3158. doi:10.1016/S0040-4039(01)81851-7.
- Chan, Eunice; Putt, Sterling R.; Showalter, H. D. Hollis; Baker, David C. (1982). "Total synthesis of (8R)-3-(2-deoxy-.beta.-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazodiazepin-8-ol (pentostatin), the potent inhibitor of adenosine deaminase". The Journal of Organic Chemistry. 47 (18): 3457–3464. doi:10.1021/jo00139a015.
- Baker, David C.; Putt, Sterling R.; Showalter, H. D. Hollis (1983). "Studies related to the total synthesis of pentostatin. Approaches to the synthesis of (8R)-3,6,7,8-tetrahydroimidazo-diazepin-8-ol andN-3 alkyl congeners". Journal of Heterocyclic Chemistry. 20 (3): 629–634. doi:10.1002/jhet.5570200324.
- Tunac, J. B.; Underhill, M. (1985). "2'-Chloropentostatin: Discovery, fermentation and biological activity.". The Journal of Antibiotics. 38 (10): 1344–1349. doi:10.7164/antibiotics.38.1344.
- Kusakabe Hitoshi & Kodama Kenjirou (+4), JPS52128292 (1977-10-27 to Yamasa Shoyu Kk); CA, 88,73015
- GB2005661 idem David C. Baker & Sterling R. Putt, US4117229 (1978 to Warner Lambert Co LLC).
- David C. Baker & Sterling R. Putt, US4195176 (1980 to Warner Lambert Co LLC).
- Pasit Phiasivongsa, Sanjeev Redkar, WO2005027838 (2005 to Supergen, Inc.).
- Oleksandr Zabudkin, Christian Schickaneder, Iaroslav Matviienko, Volodymyr Sypchenko, WO2017005784 (2017 to Synbias Pharma Ag).
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |