| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Diepoxybutane" – news · newspapers · books · scholar · JSTOR (December 2024) (Learn how and when to remove this message) |
| |
| Names | |
|---|---|
| Preferred IUPAC name 2,2′-Bioxirane | |
| Other names 1,1′-Bi; 1,2:3,4-Diepoxybutane; 1,3-Butadiene diepoxide; Bioxirane; Butadiene dioxide; Butane diepoxide; Dioxybutadiene | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| Abbreviations | DEB |
| Beilstein Reference | 79831 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.014.527 |
| EC Number |
|
| PubChem CID | |
| UNII |
|
| UN number | 3384 3082 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H6O2 |
| Molar mass | 86.090 g·mol |
| Density | 1.113 g/cm (18 °C) |
| Melting point | 4 °C (39 °F; 277 K) |
| Boiling point | 138 °C (280 °F; 411 K) |
| Solubility in water | Miscible |
| Vapor pressure | 0.52 kPa (at 20 °C) |
| Hazards | |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H226, H301, H310, H311, H314, H330, H340, H350 |
| Precautionary statements | P201, P202, P210, P233, P240, P241, P242, P243, P260, P262, P264, P270, P271, P280, P281, P284, P301+P310, P301+P330+P331, P302+P350, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P312, P320, P321, P322, P330, P361, P363, P370+P378, P403+P233, P403+P235, P405, P501 |
| Flash point | 46 °C (115 °F; 319 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
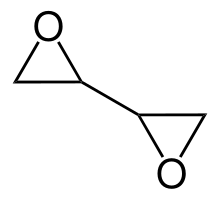
Diepoxybutane (also known as butane diepoxide, butadiene diepoxide, or 1,2:3,4-diepoxybutane) is an epoxide which is a colorless liquid at room temperature. Epoxides are very reactive due to ring strain and diepoxybutane contains two of these groups, so it is highly reactive, more than other ethers. It is hydrophilic, very flammable and easily ignited by heat or sparks.
Diepoxybutane is used as a chemical intermediate, as a cross-linking agent for polymers and textiles, and as a preservative.
Structure, reactivity, synthesis
Diepoxybutane occurs as several enantiomers and a meso form.
Diepoxybutane polymerizes in the presence of catalysts or when heated. These polymerization reactions can be violent.
Other Uses
In research diepoxybutane is used as a chemical intermediate, and in medicine for the diepoxybutane (DEB test) to screen for Fanconi anemia (FA) among patients with bone marrow failure syndromes.
Although many chemicals are capable of DNA crosslinking, the DEB test is used because it gives fewer false negatives and positives than other chemicals.
Toxicity
Effect on humans
Diepoxybutane irritates the nose, throat and lungs, causing coughing and shortness of breath. Skin exposure can cause chemical burns. Longer exposure periods can cause pulmonary edema, and damage to the liver and kidneys.
Carcinogenicity
- IARC Carcinogen - Class 1: International Agency for Research on Cancer classifies chemicals as established human carcinogens.
- NTP Carcinogen - Reasonably anticipated to be a human carcinogen.
Effect on animals
It is experimentally shown that diepoxybutane can cause tumors in rodent species at several different tissue sites and by several different exposure routes. Dermal contact with diepoxybutane caused skin tumors in mice. Injection of diepoxybutane into mice and rats caused lung tumors. Furthermore, inhalation exposure to diepoxybutane caused benign Harderian-gland tumors in mice and also increased the size of benign or malignant tumors of the nasal cavity.
References
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Diepoxybutane Report on Carcinogens, Twelfth Edition (2011)
- "Diepoxybutane". CAMEO Chemicals. NOAA. Retrieved 2023-03-19.
- "Diepoxybutane Test". www.datadictionary.nhs.uk. Retrieved 2023-03-19.
- Auerbach, AD; Tserelov, AM (1 April 2015). "Diagnosis of Fanconi anemia by diepoxybutane analysis". Current protocols in human genetics. 85: 8.7.1-8.7.17. doi:10.1002/0471142905.hg0807s85. PMC 4408609. PMID 25827349. Retrieved 30 December 2024.
- New Jersey Department of Health and Senior Services (2000). Hazardous Substances Fact Sheet. 984-2202 (609). Retrieved 2023-03-20. https://nj.gov/health/eoh/rtkweb/documents/fs/0685.pdf.