Specific dynamic action (SDA), also known as thermic effect of food (TEF) or dietary induced thermogenesis (DIT), is the amount of energy expenditure above the basal metabolic rate due to the cost of processing food for use and storage. Heat production by brown adipose tissue which is activated after consumption of a meal is an additional component of dietary induced thermogenesis. The thermic effect of food is one of the components of metabolism along with resting metabolic rate and the exercise component. A commonly used estimate of the thermic effect of food is about 10% of one's caloric intake, though the effect varies substantially for different food components. For example, dietary fat is very easy to process and has very little thermic effect, while protein is hard to process and has a much larger thermic effect.
Factors that affect the thermic effect of food
The thermic effect of food is increased by both aerobic training of sufficient duration and intensity or by anaerobic weight training. However, the increase is marginal, amounting to 7-8 calories per hour. The primary determinants of daily TEF are the total caloric content of the meals and the macronutrient composition of the meals ingested. Meal frequency has little to no effect on TEF; assuming total calorie intake for the days are equivalent.
Although some believe that TEF is reduced in obesity, discrepant results and inconsistent research methods have failed to validate such claims.
The mechanism of TEF is unknown. TEF has been described as the energy used in the distribution of nutrients and metabolic processes in the liver, but a hepatectomized animal shows no signs of TEF and intravenous injection of amino acids results in an effect equal to that of oral ingestion of the same amino acids.
Types of foods
The thermic effect of food is the energy required for digestion, absorption, and disposal of ingested nutrients. Its magnitude depends on the composition of the food consumed:
- Carbohydrates: 5 to 15% of the energy consumed
- Protein: 20 to 30%
- Fats: at most 5 to 15%
Raw celery and grapefruit are often claimed to have negative caloric balance (requiring more energy to digest than recovered from the food), presumably because the thermic effect is greater than the caloric content due to the high fibre matrix that must be unraveled to access their carbohydrates. However, there has been no research carried out to test this hypothesis and a significant amount of the thermic effect depends on the insulin sensitivity of the individual, with more insulin-sensitive individuals having a significant effect while individuals with increasing resistance have negligible to zero effects.
The Functional Food Centre at Oxford Brookes University conducted a study into the effects of chilli peppers and medium-chain triglycerides (MCT) on Diet Induced Thermogenesis (DIT). They concluded that "adding chilli and MCT to meals increases DIT by over 50% which over time may accumulate to help induce weight loss and prevent weight gain or regain".
Australia's Human Nutrition conducted a study on the effect of meal content in lean women's diets on the thermic effect of food and found that the inclusion of an ingredient containing increased soluble fibre and amylose did not reduce spontaneous food intake but rather was associated with higher subsequent energy intakes despite its reduced glycaemic and insulinemic effects.
Measuring TEF
The thermic effect of food should be measured for a period of time greater than or equal to five hours.
The American Journal of Clinical Nutrition published that TEF lasts beyond six hours for the majority of people.
References
- ^ Denzer, CM; JC Young (September 2003). "The effect of resistance exercise on the thermic effect of food". International Journal of Sport Nutrition and Exercise Metabolism. 13 (3): 396–402. doi:10.1123/ijsnem.13.3.396. PMID 14669938.
- Cannon, B.; Nedergaard, J. (2004). "Brown Adipose Tissue: Function and Physiological Significance". Physiological Reviews. 84 (1): 277–359. doi:10.1152/physrev.00015.2003. PMID 14715917.
- Christensen, Peter. "What is the thermic effect of food?". Retrieved March 28, 2005. Archived November 17, 2007, at the Wayback Machine
- Granata, G. P.; Brandon, L. J. (2002). "The Thermic Effect of Food and Obesity: Discrepant Results and Methodological Variations". Nutrition Reviews. 60 (8): 223–233. doi:10.1301/002966402320289359. PMID 12199298.
- ^ Chaprapani U. and Satyanaryana. Biochemistry, 4th Ed. Elsevier India, 2013 ISBN 9788131236017
- Edward F. Goljan (2013). Rapid Review Pathology. Elsevier Health Sciences. p. 174. ISBN 978-0-323-08787-2.
- ^ Glickman, N; Mitchell, HH (Jul 10, 1948). "The total specific dynamic action of high-protein and high-carbohydrate diets on human subjects" (PDF). The Journal of Nutrition. 36 (1): 41–57. doi:10.1093/jn/36.1.41. PMID 18868796.
- Halton, T. L.; Hu, F. B. (2004). "The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review". J Am Coll Nutr. 23 (5): 373–85. doi:10.1080/07315724.2004.10719381. PMID 15466943. S2CID 28136289.
- Segal, K. R.; Albu, J.; Chun, A.; Edano, A.; Legaspi, B.; Pi-Sunyer, F. X. (1992). "Independent effects of obesity and insulin resistance on postprandial thermogenesis in men". Journal of Clinical Investigation. 89 (3): 824–833. doi:10.1172/JCI115661. PMC 442927. PMID 1541675.
- Camastra, S.; Bonora, E.; Del Prato, S.; Rett, K.; Weck, M.; Ferrannini, E. (1999). "Effect of obesity and insulin resistance on resting and glucose-induced thermogenesis in man. EGIR (European Group for the Study of Insulin Resistance)". International Journal of Obesity and Related Metabolic Disorders. 23 (12): 1307–1313. doi:10.1038/sj.ijo.0801072. PMID 10643689.
- Clegg, M. E.; Golsorkhi, M.; Henry, C. J. (2012). "Combined medium-chain triglyceride and chilli feeding increases diet-induced thermogenesis in normal-weight humans". European Journal of Nutrition. 52 (6): 1579–1585. doi:10.1007/s00394-012-0463-9. PMID 23179202. S2CID 45846650.
- JKeogh, J. B.; Lau, C. W. H.; Noakes, M.; Bowen, J.; Clifton, P. M. (2006). "Effects of meals with high soluble fibre, high amylose barley variant on glucose, insulin, satiety and thermic effect of food in healthy lean women". European Journal of Clinical Nutrition. 61 (5): 597–604. doi:10.1038/sj.ejcn.1602564. PMID 17164830.
- ^ Reed, GW; Hill, JO (Feb 1996). "Measuring the thermic effect of food". The American Journal of Clinical Nutrition. 63 (2): 164–9. doi:10.1093/ajcn/63.2.164. PMID 8561055.
Further reading
- Glickman, N; Mitchell, HH (Jul 10, 1948). "The total specific dynamic action of high-protein and high-carbohydrate diets on human subjects" (PDF). The Journal of Nutrition. 36 (1): 41–57. doi:10.1093/jn/36.1.41. PMID 18868796.
| Metabolism, catabolism, anabolism | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||
| Energy metabolism |
| ||||||||||||||||||||||||||||||||
| Specific paths |
| ||||||||||||||||||||||||||||||||
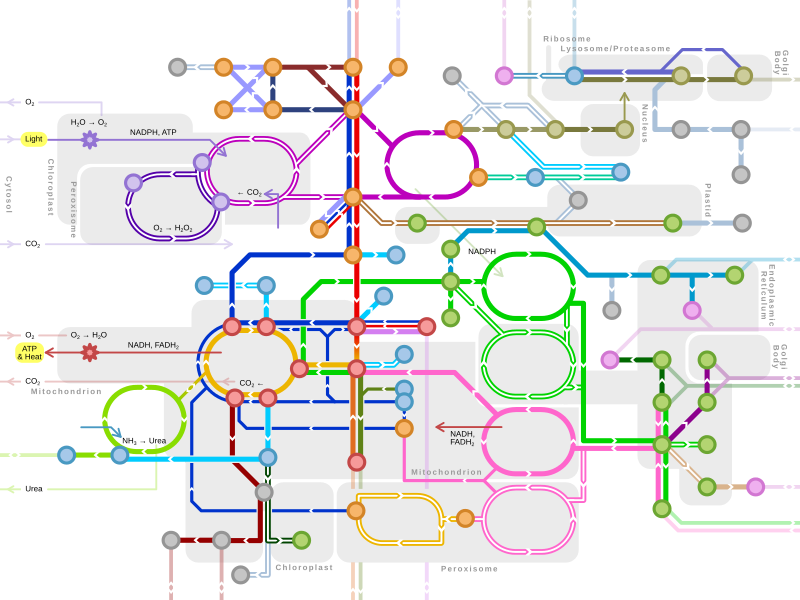
| Metabolism map | ||
|---|---|---|
Single lines: pathways common to most lifeforms. Double lines: pathways not in humans (occurs in e.g. plants, fungi, prokaryotes). | ||
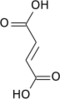
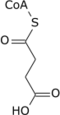
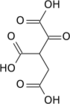
| Citric acid cycle metabolic pathway | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
| Metabolism: Citric acid cycle enzymes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cycle | |||||||||
| Anaplerotic |
| ||||||||
| Mitochondrial electron transport chain/ oxidative phosphorylation |
| ||||||||