| |
| Names | |
|---|---|
| Other names
Dimolybdenum tetraacetate, tetra(aceto) dimolybdenum, Molybdenum(II) acetate dimer | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.034.611 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H12Mo2O8 |
| Molar mass | 428.1010 g/mol |
| Appearance | Yellow solids |
| Boiling point | decomposes |
| Solubility in water | not soluble |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P222, P231, P235, P305+P351+P338, P422, P501 |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
| Related compounds | Copper(II) acetate Chromium(II) acetate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
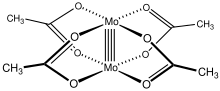
Molybdenum(II) acetate is a coordination compound with the formula Mo2(O2CCH3)4. It is a yellow, diamagnetic, air-stable solid that is slightly soluble in organic solvents. Molybdenum(II) acetate is an iconic example of a compound with a metal-metal quadruple bond.
Structure
Like several other transition metal carboxylate complexes, Mo2(O2CCH3)4 adopts a Chinese lantern structure. Each Mo(II) center in Mo2(O2CCH3)4 has four d valence electrons. These eight d-electrons form one σ, two π bonds, and one δ bond, creating a bonding electron configuration of σπδ. Each of these bonds are formed by the overlapping of pairs of d orbitals. The four acetate groups bridge the two metal centers. The Mo-O bond between each Mo(II) center and O atom from acetate has a distance of 2.119 Å, and the Mo-Mo distance between the two metal centers is 2.0934 Å.
Preparation
Mo2(O2CCH3)4 is prepared by treating molybdenum hexacarbonyl (Mo(CO)6) with acetic acid. The process strips CO ligands from the hexacarbonyl and results in the oxidation of Mo(0) to Mo(II).
- 2 Mo(CO)6 + 4 HO2CCH3 → Mo2(O2CCH3)4 + 12 CO + 2 H2
Trinuclear clusters are byproducts.
The reaction of HO2CCH3 and Mo(CO)6 was first investigated by Bannister et al. in 1960. At the time, quadruple metal-metal bonds had not yet been discovered, so these authors proposed that Mo(O2CCH3)2 was tetrahedral. This perspective changed with Mason's characterization.
Applications
Mo2(O2CCH3)4 is generally used as an intermediate compound in a process to form other quadruply bonded molybdenum compounds. The acetate ligands can be replaced to give new compounds such as and Mo2Cl44.
References
- "Molybdenum (CAS Number 14221-06-8) : Strem Product Catalog". www.strem.com. Retrieved 27 December 2021.
- ^ Girolami, G. S.; Rauchfuss, T. B. and Angelici, R. J., "Synthesis and Technique in Inorganic Chemistry third edition", University Science Books: Mill Valley, CA, 1999, ISBN 0-935702-48-2
- Cotton, F. A.; Hillard, E.A.; Murillo, C. A.; Zhou, H.-C. "After 155 Years, A Crystalline Chromium Carboxylate with a Supershort Cr-Cr Bond" J. Am. Chem. Soc., 2000, 122, 416-417. doi:10.1021/ja993755i.
- Blaudeau, J. P.; Pitzer, R. M. “ Ab Initio Studies of Ligand Effects on the Metal-Metal Bond in Dimolybdenum Complexes” J.Phys. Chem. 1994, vol. 98, pp. 4575-4579.
- Brignole, A.G.; Cotton, F.A., “Rhenium and Molybdenum compounds containing quadruple compounds” Inorg. Synth. 1972, volume 13, pp. 81-89. doi:10.1002/9780470132449.ch15
- Pence, L. E.; Weisgerber, A. M.; Maounis, F.A.; “Synthesis of Molybdenum-Molybdenum Quadruple Bonds” J. Chem. Educ., 1999, vol. 76, 404-405.
- Bino, A.; Cotton, F.A.; Dori, A.; J. Am. Chem. Soc. 1981, vol. 103, pp. 243-244. “A Aqueous New Chemistry of Organometallic, Trinuclear Cluster Compounds of Molybdenum”.
- Bannister, E.; Wikinson, G. “Molybdenum(II) carboxylates” Chem. Ind. 1960, 319.
- Stephenson, T.A.; Bannister, E.; Wilkinson, G. “Molybdenum(II) Carboxylates” J. Chem. Soc., 1964, pp. 2538. doi:10.1039/JR9640002538
- D. Lawton, R. Mason "The Molecular Structure of Molybdenum(II) Acetate"J. Am. Chem. Soc. 1965, vol 87, pp 921–922. doi:10.1021/ja01082a046
- Tsai, Y.C.; Chen H.Z.; Chang, C.C.; Yu, J.K.; Lee, G.H.; Wang, Y.; Kuo, T.S. “Journey from Mo-Mo Quadruple Bonds to Quintuple Bonds” J. Am. Chem. Soc., 2009, 131, 12534-12535. doi:10.1021/ja905035f
- Handa, M.; Mikuriya, M.; Kotera, T.; Yamada, K.; Nakso, T.; Matsumoto, H.; Kasuga, K. “Linear Chain Compounds of Molybdenum(II) Acetate Linked by Pyazine, 4,4’-Bipyridine, and 1,4- Diazabicyclooctane” Bull. Chem. Soc. Jpn., 1995,68, 2567-2572.
| Molybdenum compounds | |
|---|---|
| Mo(0) | |
| Mo(II) | |
| Mo(III) | |
| Mo(IV) | |
| Mo(V) | |
| Mo(VI) | |
| Acetyl halides and salts of the acetate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||