
| |
| Names | |
|---|---|
| IUPAC name 1,3-diphosphonia-2,4-diboranuidacyclobutane | |
| Identifiers | |
| 3D model (JSmol) | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | B2H8P2 |
| Molar mass | 91.63 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
1,3-Diphospha-2,4-diboretanes, or B2P2, is a class of 4-member cyclic compounds of alternating boron and phosphorus atoms. They are often found as dimers during the synthesis of boraphosphenes (RB=PR'). Compounds can exhibit localized singlet diradical character (diradicaloid) between the boron atoms in the solution and solid state.
Synthesis
The first suggested synthesis of a diphosphadiboretane compound was with phosphinoarylboranes dimerization in 1961. Concurrently, a diphosphadiboretane was determined to be produced from the reaction with phosphinosilanes and boron halides. The following thermolysis treatment formed boraphosphenes by cleavage of the σ P-Si bonds. Dimerization of these compounds are highly favored (upward to 90 kcal mol), which results in isolated diphosphadiboretanes. Alternative routes of synthesis utilizing facile leaving groups have also been discovered including hydrogen halide and organylphosphane elimination to obtain the monomer precursors. Treatment with boron halides with lithium phosphides have also been found to lead to monomers which then dimerize into the diphosphadiboretanes.
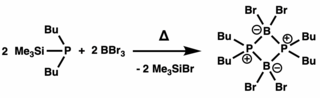
In 2002, a direct synthesis to a diphosphadiboretanediyl, a diphosphadiboretane derivative, was accomplished using 1,2-dichlorodiborane and a lithium phosphide reagent.

In 2021, it was found that the phosphination of diboryne using diphosphate would slowly convert to diphosphadiboretane in solution.

Structure and bonding

X-ray crystallography
Diphosphadiboretanes can be isolated as single crystals suitable for x-ray crystallography. The structure is found to be planar, with a near-square geometry. The B-B distance is found to be ranging from 2.57-2.71 Å, indicating a long B-B single bond. The phosphorus atoms adopt pyramidal geometries, which are preferred over a planar geometry and prevents π-bonding with the boron atoms.
Planar and bicyclic structure
The diphosphadiboretanediyl derivatives have a wider structural range, adopting planar and bicyclo geometries, similar to diphosphacylobutanediyls. These geometries can be modulated by changing the substituents on the boron or phosphorus atoms. This conversion is accompanied by lengthening and shortening of the B-B bond, respectively. Addition of more steric, protecting groups on the boron atoms and less steric groups on the phosphorus atoms favor the bicyclo structure. The latter is due to reduction of 1,3-diaxial strain. Coplanar π-bonding to the boron atom and addition of aromatic groups to the ring favors the bicyclo structure with a B-B bond length shorten down to 1.83 Å.
Diradical

In the planar, diphosphadiboretanediyl structures, the substantially long B-B bond can be considered to experience homolytic cleavage to form a localized singlet diradicaloid between the boron atoms. The singlet state energy can be up to 20-35 kcal mol more stabilized than the triplet state. Addition of π-donors on the boron atom is found to increase the singlet-triplet gap, while π-acceptors decrease it. The bicyclo structure, with a shorter B-B bond, is considered to have less diradical character and more of a closed-shell system.
Frontier orbitals
The HOMO of these diphosphadiboretanediyls consists of a π-orbital with strong boron p-orbital character. This orbital is stabilized by the presence of π-overlap between the trans-boron atoms (through-space interactions) as well as through-bond contributions by the σ* P-R orbitals. Due to the presence of the through-space interaction, computational studies have determined these compounds to have significantly less diradical character than traditional organic diradicals. The LUMO is the antibonding π* B-B orbital.

Reactivity
Transition metals
Diphosphadiboretanes have been shown to behave as ligands to metal-carbonyl complexes, forming boron-mononuclear, boron-binuclear, η, and η architectures with displacement of CO ligands. If steric, amino-containing substituents on the boron are used, then a cycloreversion reaction happens. This results in the production of metal-stabilized boraphosphene. Cage compounds (B2P2M, M = Pd, Pt) have also be reported with η coordination to the P atoms.

Oxidation
Synthesis of diphosphadiboretane from diboryne offers a two electron-rich compound, compared to diphosphadiboretanediyls. These electrons can be readily oxidized, affording the radical cation compound and diphsophadiboretanediyl compound. The radical cation is found to exhibit triplet state by electronic paramagnetic resonance, accompanying with a shortening of the B-B bond (2.63 Å) and slight perturbance of planar, square structure. The diphosphadiboretanediyl compound is found to have a B-B bond of 2.12 Å and the transition to the bicyclo structure. Natural bond orbital analysis of phosphadiboretanediyl compound indicates a bent B-B bond with a small 3 kcal mol stabilization energy compared to the planar structure.

Diradical activity
Diphosphadiboretanediyls exhibits reactivity similar to radicals, behaving as diradical intermediates. The compounds have been shown to perform chloride abstraction from deuterated chloroform, trans-addition of trimethyl tin hydride, and formation of bicyclo structure with selenium. Reactivity is displayed towards bromotrichloromethane where radical activity induces formation of boron-containing spiro-compound from the tert-butyl group. This cyclic formation maintains the diphosphadiboretane ring structure.

References
- ^ Kölle, Peter; Linti, Gerald; Nöth, Heinrich; Wood, Gary L.; Narula, Chaitanya K.; Paine, Robert T. (May 1988). "Contributions to the chemistry of boron, 188. Synthesis and Structures of New 1,3,2,4-Diphosphadiboretanes". Chemische Berichte. 121 (5): 871–879. doi:10.1002/cber.19881210510. ISSN 0009-2940.
- ^ Scheschkewitz, David; Amii, Hideki; Gornitzka, Heinz; Schoeller, Wolfgang W.; Bourissou, Didier; Bertrand, Guy (2002-03-08). "Singlet Diradicals: from Transition States to Crystalline Compounds". Science. 295 (5561): 1880–1881. Bibcode:2002Sci...295.1880S. doi:10.1126/science.1068167. ISSN 0036-8075. PMID 11884750. S2CID 11482396.
- Coates, G. E.; Livingstone, J. G. (1961). "203. Aminodiarylboranes and their phosphorus and arsenic analogues". Journal of the Chemical Society (Resumed): 1000–1008. doi:10.1039/jr9610001000. ISSN 0368-1769.
- Nöth, Heinrich; Schrägle, Wolfgang (1961-07-01). "Notizen: Über eine neue Methode zur Darstellung von Bor-Phosphor-Verbindungen". Zeitschrift für Naturforschung B. 16 (7): 473–474. doi:10.1515/znb-1961-0715. ISSN 1865-7117. S2CID 94179759.
- ^ Nöth, Heinrich; Schrägle, Wolfgang (February 1965). "Beiträge zur Chemie des Bors, XXXII. Zur Synthese dimerer und trimerer Phosphino-borane". Chemische Berichte. 98 (2): 352–362. doi:10.1002/cber.19650980205. ISSN 0009-2940.
- ^ Arif, Atta M.; Cowley, Alan H.; Pakulski, Marek; Power, John M. (1986). "Diphospha- and diarsa-diboretanes. Four-membered rings containing boron and phosphorus or arsenic". Journal of the Chemical Society, Chemical Communications (11): 889–890. doi:10.1039/c39860000889. ISSN 0022-4936.
- ^ Brückner, Tobias; Fantuzzi, Felipe; Stennett, Tom E.; Krummenacher, Ivo; Dewhurst, Rian D.; Engels, Bernd; Braunschweig, Holger (2021-06-07). "Isolation of Neutral, Mono-, and Dicationic B 2 P 2 Rings by Diphosphorus Addition to a Boron−Boron Triple Bond". Angewandte Chemie International Edition. 60 (24): 13661–13665. doi:10.1002/anie.202102218. ISSN 1433-7851. PMC 8252364. PMID 33844394.
- ^ Rodriguez, Amor; Präsang, Carsten; Gandon, Vincent; Bourg, Jean-Baptiste; Bertrand, Guy (2005-12-01). "Stable Singlet Diradicals Based on Boron and Phosphorus". In Lattman, Michael; Kemp, Richard A. (eds.). Modern Aspects of Main Group Chemistry. ACS Symposium Series. Vol. 917. Washington, DC: American Chemical Society. doi:10.1021/bk-2005-0917.ch006. ISBN 978-0-8412-3926-5.
- Schoeller, Wolfgang W.; Rozhenko, Alexander; Bourissou, Didier; Bertrand, Guy (2003-08-04). "On the Electronic Structures of the 1,3-Diboracyclobutane-1,3-diyls and Their Valence Isomers with a B2E2 Skeleton (E=N, P, As)". Chemistry - A European Journal. 9 (15): 3611–3617. doi:10.1002/chem.200204508. PMID 12898688.
- Jung, Yousung; Head-Gordon, Martin (2003-09-01). "Controlling the Extent of Diradical Character by Utilizing Neighboring Group Interactions". The Journal of Physical Chemistry A. 107 (38): 7475–7481. Bibcode:2003JPCA..107.7475J. doi:10.1021/jp034467i. ISSN 1089-5639.
- Cheng, Mu-Jeng; Hu, Ching-Han (2003-05-10). "B 2 P 2 rings: through-space π bond or stable diradical? A theoretical study". Molecular Physics. 101 (9): 1319–1323. Bibcode:2003MolPh.101.1319C. doi:10.1080/0026897031000092995. ISSN 0026-8976. S2CID 98739709.
- Kaufmann, Bernhard; Nöth, Heinrich; Paine, Robert T.; Polborn, Kurt; Thomann, Martina (October 1993). "Carbonyl-Metal Complexes of Diphosphadiboretane (mesB-P t Bu) 2 and of Triphosphatriborinanes (mesBP t Bu) 3". Angewandte Chemie International Edition in English. 32 (10): 1446–1448. doi:10.1002/anie.199314461.
- Chen, Tuqiang; Duesler, Eileen N.; Paine, Robert T.; Nöth, Heinrich (1998-02-01). "Formation of Cage Compounds Containing Boron, Phosphorus, and Transition Metal Atoms". Inorganic Chemistry. 37 (3): 490–495. doi:10.1021/ic970965s. ISSN 0020-1669. PMID 11670299.