| |
| Names | |
|---|---|
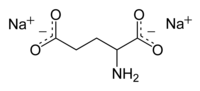
| IUPAC name Disodium 2-aminopentanedioate | |
| Other names DSG | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H7NNa2O4 |
| Molar mass | 191.09 g/mol |
| Appearance | white crystalline powder |
| Odor | practically odorless |
| Boiling point | 225 °C (437 °F; 498 K) (decomposes) |
| Solubility in water | 73.9 g/100 mL (25 °C) |
| Solubility | sparingly soluble in alcohol |
| Acidity (pKa) | 6.8 |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 16600 mg.mg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Disodium glutamate, abbreviated DSG, (Na2C5H7NO4) is a sodium salt of glutamic acid. It is used as a flavoring agent to impart umami flavor.
Formation
Disodium glutamate can be produced by neutralizing glutamic acid with two molar equivalents of sodium hydroxide (NaOH).
See also
References
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |