Pharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ditropan, Gelnique, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682141 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, transdermal |
| Drug class | Antimuscarinic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 91–93% |
| Metabolites | N-Desethyloxybutynin |
| Elimination half-life | 12.4–13.2 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.590 |
| Chemical and physical data | |
| Formula | C22H31NO3 |
| Molar mass | 357.494 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Oxybutynin, sold under the brand name Ditropan among others, is an anticholinergic medication primarily used to treat overactive bladder. It is widely considered a first-line therapy for overactive bladder due to its well-studied side effect profile, broad applicability, and continued efficacy over long periods of time. It works similar to tolterodine, darifenacin, and solifenacin, although it is usually preferred over these medications. It is sometimes used off-label for treatment of hyperhidrosis, or excessive sweating. It has also been used off-label to treat bedwetting in children, but this use has declined, as it is most likely ineffective in this role. It is taken by mouth or applied to the skin.
Common side effects include dry mouth, constipation, dizziness, trouble sleeping, and urinary tract infections. Serious side effects may include urinary retention and an increased risk of heat stroke. Use in pregnancy appears safe but has not been well studied while use in breastfeeding is of unclear safety. It is an antimuscarinic and works by blocking the effects of acetylcholine on smooth muscle.
Oxybutynin was approved for medical use in the US in 1975. It is available as a generic medication. In 2022, it was the 110th most commonly prescribed medication in the United States, with more than 5 million prescriptions.
Medical use
Oxybutynin is used in the form of instant-release capsules, extended-release capsules, or transdermal (topical) products. All of these are considered safe and effective options for treatment of detrusor muscle-mediated overactive bladder. Extended-release formulations decrease the number of weekly incontinence episodes by an average of 90% compared to an untreated state. Some studies have identified advantages of transdermal oxybutynin over capsules, finding decreased frequency of incontinence episodes and increased average voided volume of urine.

Oxybutynin has been established through head-to-head trials as a more effective for overactive bladder than tolterodine, another anticholinergic medication. Specifically, the extended-release form of oxybutynin was found to have greater effect in both the short- and long-term. However, oxybutynin is not selective for the bladder like tolterodine, and thus has a wider range of side effects. Tolterodine and other anticholinergics are primarily used when clinicians and patients want to reduce the side effect profile.
Because both drugs have been studied extensively and shown relatively high efficacy, both oxybutynin and tolterodine are considered first-line treatments for overactive bladder. They are thus the typical choices for initial treatment of the condition. The choice of initial therapy often comes down to whether a patient prefers somewhat higher efficacy (oxybutynin) or somewhat reduced side effects (tolterodine).
Hyperhidrosis
Since the 2010s, oxybutynin has increasingly been used to treat hyperhidrosis (excessive sweating). Numerous studies have identified concrete benefits of the drug in treating this condition, but have not identified appropriate dosing or the full spectrum of possible side effects, although dry mouth is seemingly infrequent in patients with hyperhidrosis. Until further clinical trials can be conducted, oxybutynin is only used as an off-label medication for hyperhidrosis (as of 2024).
Adverse effects
Common adverse effects that are associated with oxybutynin and other anticholinergics include: dry mouth, difficulty in urination, constipation, blurred vision, drowsiness, and dizziness. Anticholinergics have also been known to induce delirium.
Oxybutynin's tendency to reduce sweating can be dangerous. Reduced sweating increases the risk of heat exhaustion and heat stroke in apparently safe situations where normal sweating keeps others safe and comfortable. Adverse effects of elevated body temperature are more likely for the elderly and for those with health issues, especially multiple sclerosis.
N-Desethyloxybutynin is an active metabolite of oxybutynin that is thought responsible for much of the adverse effects associated with the use of oxybutynin. N-Desethyloxybutynin plasma levels may reach as much as six times that of the parent drug after administration of the immediate-release oral formulation. Alternative dosage forms have been developed in an effort to reduce blood levels of N-desethyloxybutynin and achieve a steadier concentration of oxybutynin than is possible with the instant-release form. The long-acting formulations also allow once-daily administration instead of the twice-daily dosage required with the immediate-release form. The transdermal patch, in addition to the benefits of the extended-release oral formulations, bypasses the first-pass hepatic effect that the oral formulations are subject to.
Contraindications
| This section does not cite any sources. Please help improve this section by adding citations to reliable sources. Unsourced material may be challenged and removed. (March 2024) (Learn how and when to remove this message) |
Oxybutynin chloride is contraindicated in patients with untreated narrow angle glaucoma, and in patients with untreated narrow anterior chamber angles—since anticholinergic drugs may aggravate these conditions. It is also contraindicated in partial or complete obstruction of the gastrointestinal tract, hiatal hernia, gastroesophageal reflux disease, paralytic ileus, intestinal atony of the elderly or debilitated patient, megacolon, toxic megacolon complicating ulcerative colitis, severe colitis, and myasthenia gravis. It is contraindicated in patients with obstructive uropathy and in patients with unstable cardiovascular status in acute hemorrhage. Oxybutynin chloride is contraindicated in patients who have demonstrated hypersensitivity to the product.
Pharmacology
Sources say the drug is absorbed within one hour and has an elimination half-life of 2 to 5 hours. There is a wide variation among individuals in the drug's concentration in blood. This, and its low concentration in urine, suggest that it is eliminated through the liver.
Chemistry
Oxybutynin contains one stereocenter. Commercial formulations are sold as the racemate. The (R)-enantiomer is a more potent anticholinergic than either the racemate or the (S)-enantiomer, which is essentially without anticholinergic activity at doses used in clinical practice. However, (R)-oxybutynin administered alone offers little or no clinical benefit above and beyond the racemic mixture. The other actions (calcium antagonism, local anesthesia) of oxybutynin are not stereospecific. (S)-Oxybutynin has not been clinically tested for its spasmolytic effects, but may be clinically useful for the same indications as the racemate, without the unpleasant anticholinergic side effects.
| Enantiomers of oxybutynin | |
|---|---|
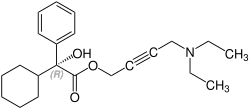 CAS-Number: 119618-21-2 |
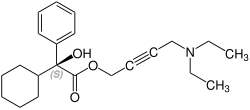 CAS-Number: 119618-22-3 |
Society and culture
Brand names
Oxybutynin is available by mouth in generic formulation and under the brand names Ditropan, Lyrinel XL, Ditrospam, Kentera, and Aquiette, as a transdermal patch under the brand name Oxytrol, and as a topical gel under the brand name Gelnique.
Research
A large study linked the development of dementia in those over 65 to the use of oxybutynin, due to its anticholinergic properties.
References
- "Ditropan XL (oxybutynin chloride) Extended Release Tablets for oral use Initial U.S. Approval: 1975". DailyMed. Retrieved June 17, 2024.
- "Gelnique- oxybutynin chloride gel". DailyMed. March 1, 2019. Retrieved June 17, 2024.
- "Oxytrol- oxybutynin patch". DailyMed. May 29, 2024. Retrieved June 17, 2024.
- "Oxytrol for Women- oxybutynin patch". DailyMed. August 13, 2016. Retrieved June 17, 2024.
- "Kentera EPAR". European Medicines Agency (EMA). June 15, 2004. Retrieved October 12, 2024.
- ^ "Oxybutynin Chloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved March 3, 2019.
- "Oxybutynin Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved March 3, 2019.
- British National Formulary: BNF 76 (76th ed.). Pharmaceutical Press. 2018. ISBN 978-0-85711-338-2.
- "The Top 300 of 2022". ClinCalc. Archived from the original on August 30, 2024. Retrieved August 30, 2024.
- "Oxybutynin Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved August 30, 2024.
- ^ Diokno A, Ingber M (November 2006). "Oxybutynin in detrusor overactivity". The Urologic Clinics of North America. Overactive Bladder. 33 (4): 439–45, vii. doi:10.1016/j.ucl.2006.06.003. PMID 17011379.
- Baldwin CM, Keating GM (2009). "Transdermal oxybutynin". Drugs. 69 (3): 327–337. doi:10.2165/00003495-200969030-00008. PMID 19275276. S2CID 33534909.
- Loloi J, Clearwater W, Schulz A, Suadicani SO, Abraham N (May 2022). "Medical Treatment of Overactive Bladder". The Urologic Clinics of North America. Urologic Pharmacology. 49 (2): 249–261. doi:10.1016/j.ucl.2021.12.005. PMID 35428431.
- White N, Iglesia CB (March 2016). "Overactive Bladder". Obstetrics and Gynecology Clinics of North America. Medical and Advanced Surgical Management of Pelvic Floor Disorders. 43 (1): 59–68. doi:10.1016/j.ogc.2015.10.002. PMID 26880508.
- Cruddas L, Baker DM (June 2017). "Treatment of primary hyperhidrosis with oral anticholinergic medications: a systematic review". Journal of the European Academy of Dermatology and Venereology. 31 (6): 952–963. doi:10.1111/jdv.14081. PMID 27976476. S2CID 4535312.
- ^ El-Samahy M, Mouffokes A, Badawy MM, Amro S, Fayad T, Abdelwahab OA (October 2023). "Safety and efficacy of oxybutynin in patients with hyperhidrosis: systematic review and meta-analysis of randomized controlled trials". Archives of Dermatological Research. 315 (8): 2215–2226. doi:10.1007/s00403-023-02587-5. PMC 10462517. PMID 36869926.
- Mehta D, ed. (2006). British National Formulary. Vol. 51. Pharmaceutical Press. ISBN 0-85369-668-3.
- Andreasen NC, Black DW (2006). Introductory Textbook of Psychiatry. American Psychiatric Publishing Inc.
- "Oxybutynin (By mouth)". PubMed Health. U.S. National Library of Medicine. mmdn/DNX0064.
- White AT, Vanhaitsma TA, Vener J, Davis SL (June 2013). "Effect of passive whole body heating on central conduction and cortical excitability in multiple sclerosis patients and healthy controls". Journal of Applied Physiology. 114 (12): 1697–1704. doi:10.1152/japplphysiol.01119.2012. PMC 3680823. PMID 23599395.
- Reitz AB, Gupta SK, Huang Y, Parker MH, Ryan RR (November 2007). "The preparation and human muscarinic receptor profiling of oxybutynin and N-desethyloxybutynin enantiomers". Medicinal Chemistry. 3 (6): 543–545. doi:10.2174/157340607782360353. PMID 18045203.
- Zobrist RH, Schmid B, Feick A, Quan D, Sanders SW (July 2001). "Pharmacokinetics of the R- and S-enantiomers of oxybutynin and N-desethyloxybutynin following oral and transdermal administration of the racemate in healthy volunteers". Pharmaceutical Research. 18 (7): 1029–1034. doi:10.1023/a:1010956832113. PMID 11496941. S2CID 8004795.
- Oki T, Toma-Okura A, Yamada S (March 2006). "Advantages for transdermal over oral oxybutynin to treat overactive bladder: Muscarinic receptor binding, plasma drug concentration, and salivary secretion". The Journal of Pharmacology and Experimental Therapeutics. 316 (3): 1137–1145. doi:10.1124/jpet.105.094508. PMID 16282521. S2CID 30397079.
- "Oxybutynin". drugs.com. Retrieved August 30, 2012.
- ^ Douchamps J, Derenne F, Stockis A, Gangji D, Juvent M, Herchuelz A (1988). "The pharmacokinetics of oxybutynin in man". European Journal of Clinical Pharmacology. 35 (5): 515–520. doi:10.1007/bf00558247. PMID 3234461. S2CID 33628778.
- Kachur JF, Peterson JS, Carter JP, Rzeszotarski WJ, Hanson RC, Noronha-Blob L (December 1988). "R and S enantiomers of oxybutynin: pharmacological effects in guinea pig bladder and intestine". The Journal of Pharmacology and Experimental Therapeutics. 247 (3): 867–872. PMID 2849672.
- Noronha-Blob L, Kachur JF (February 1991). "Enantiomers of oxybutynin: in vitro pharmacological characterization at M1, M2 and M3 muscarinic receptors and in vivo effects on urinary bladder contraction, mydriasis and salivary secretion in guinea pigs". The Journal of Pharmacology and Experimental Therapeutics. 256 (2): 562–567. PMID 1993995.
- "DITROPAN®(oxybutynin chloride) Tablets and Syrup" (PDF). FDA. February 2008. Retrieved June 18, 2020.
- "Oxybutynin – Brand names: Ditropan, Lyrinel, Kentera". NHS UK. June 15, 2021.
- Robinson J (April 26, 2022). "Pharmacists express concerns over proposed reclassification of overactive bladder drug". The Pharmaceutical Journal.
- Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. (March 2015). "Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study". JAMA Internal Medicine. 175 (3): 401–407. doi:10.1001/jamainternmed.2014.7663. PMC 4358759. PMID 25621434.
| Urologicals, including antispasmodics (G04B) | |
|---|---|
| Acidifiers | |
| Urinary antispasmodics (primarily antimuscarinics) | |
| Other urologicals |
|
| Muscarinic acetylcholine receptor modulators | |||||
|---|---|---|---|---|---|
| mAChRsTooltip Muscarinic acetylcholine receptors |
| ||||
| Precursors (and prodrugs) | |||||