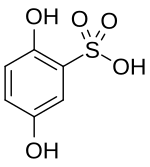
| |
| Names | |
|---|---|
| IUPAC name 2,5-Dihydroxybenzenesulfonic acid | |
| Other names Hydroquinonesulfonic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.667 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H6O5S |
| Molar mass | 190.17 g·mol |
| Related compounds | |
| Other cations | Calcium dobesilate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Dobesilic acid is a chemical compound with the molecular formula C6H6O5S. It is classified as both a phenol and a sulfonic acid.
Uses
Salts of dobesilic are used as pharmaceutical drugs. The calcium salt, calcium dobesilate, is used as a vasoprotective drug. The diethylamine salt, etamsylate, is an antihemorrhagic agent.
References
- Zhang X, Liu W, Wu S, Jin J, Li W, Wang N (January 2015). "Calcium dobesilate for diabetic retinopathy: a systematic review and meta-analysis". Science China Life Sciences. 58 (1): 101–7. doi:10.1007/s11427-014-4792-1. PMID 25528255.
- Haller H, Ji L, Stahl K, Bertram A, Menne J (2017). "Molecular Mechanisms and Treatment Strategies in Diabetic Nephropathy: New Avenues for Calcium Dobesilate-Free Radical Scavenger and Growth Factor Inhibition". BioMed Research International. 2017: 1909258. doi:10.1155/2017/1909258. PMC 5634607. PMID 29082239.
- Schulte J, Osborne J, Benson JW, Cooke R, Drayton M, Murphy J, et al. (January 2005). "Developmental outcome of the use of etamsylate for prevention of periventricular haemorrhage in a randomised controlled trial". Archives of Disease in Childhood. Fetal and Neonatal Edition. 90 (1): F31–F35. doi:10.1136/adc.2003.035790. PMC 1721806. PMID 15613570.